Iron is one of the most essential nutrients. It is not classified as either macro or microelements; it simply must be constantly present in plant nutrition. Lack of iron leads to a disruption in the production of chlorophyll in the leaves, i.e., a gradual cessation of vital important function– the process of photosynthesis.
The most surprising thing is that iron is the most common element, and it is found in sufficient quantities in the soil, but, unfortunately, in a form inaccessible to plants. The only available and easily absorbed form of the metal is iron chelate.
What is iron chelate
Chelates are highly effective microfertilizers that contain one or more microelements enclosed in a shell. As the shell disintegrates, beneficial elements are released and nourish the plants. One such fertilizer is iron chelate.
III-valent iron – Fe(III) – is found in free form in the soil. But its molecules are inactive and bring virtually no benefit to plants and are not absorbed. The divalent form of iron Fe(II) is mobile, easily and quickly absorbed, but the problem is that such iron very quickly oxidizes, turning into the trivalent form (rust).
To prevent this from happening, Fe(II) is placed in a “shell” - a chelate complex, which consists of weak organic acids (most often citric acid). Iron in the chelate shell can retain its II-valence structure for a long time until the decomposition of the chelate complex occurs. The advantages of using iron chelate are that:
- the breakdown of chelates occurs at the same rate as the absorption of iron by plants, i.e. iron oversaturation cannot occur, plants take as much as they need;
- the chelate shell breaks down into elements that do not pollute the soil and are harmless to environment– oxygen, hydrogen, carbon.
Chlorosis, its causes and treatment
Why do plants need iron chelate? Iron is responsible for the normal course of the most important process in plants - photosynthesis. A deficiency of the element leads to infection of plant organisms with chlorosis, i.e. the synthesis of chlorophyll in green leaves is disrupted.

Plants seem to be losing vitality, weaken and may even die. This manifests itself mainly on newly developing shoots as follows:
- the plates of young leaves between the veins become yellow, but the veins themselves remain bright green;
- leaves become small;
- there is an unreasonable fall of leaves and unopened buds;
- the shape of buds and flowers changes and bends;
- the edges of the leaves curl;
- apical shoots do not develop or dry out;
- the development of the root system slows down or stops, in the worst case – death of the roots.
Even one of these symptoms indicates an insufficient amount of iron in the soil. To help the plants, it is necessary to apply root or foliar feeding with an iron solution.
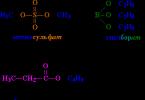
Which is better for plants - iron chelate or iron sulfate?
The most common fertilizers containing iron are chelates and sulfates. However, many gardeners are inclined to believe that iron chelate is much more effective and safer than sulfate:
- during the decomposition of Fe2 (SO4)3 fertilizer, much less divalent iron is released than active SO4 ions;
- the rate of release of Fe(II) and its absorption by plants do not coincide, so most of the useful element is lost;
- to achieve the norm of iron consumption, you will have to oversaturate the plants with sulfur, which results in sulfur poisoning;
- iron sulfate is ineffective on depleted soils, in summer time and in difficult climatic conditions.
It is much more effective to use iron chelate to feed plants.

How to use
Fruit trees - apple trees, pears, plums, peaches, cherries, lemons - suffer most from iron deficiency. In addition, microelement deficiency is noticeable in such fruit and vegetable crops as tomatoes, cucumbers, carrots, potatoes, corn, and raspberries. The most effective are foliar treatments of plants by leaves, but root watering also gives good results.
To prevent chlorosis
For preventive purposes, spray the leaves every two weeks from the appearance of new leaves until the start of flowering (but at least 2 times). To do this, prepare a solution: 5 g of iron chelate is diluted in 10 liters of water, which is used to treat plants at the rate of 1 liter per 10 m2.
For the treatment of chlorosis
5 g of chelate is dissolved in 5 liters of water to fruit trees, in 8 liters of water for vegetable crops. Treatment must be carried out at least 4 times with a break of 2 weeks. If it is necessary to treat deep chlorosis, you can water the plants at the root - 2 liters per 1 sq.m.
For indoor plants
Some types of domestic flowers are especially demanding of iron and other trace elements, as they are forced to remain in a closed substance for a long time. Iron deficiency is experienced by:
- azaleas;
- hydrangeas;
- clerodendrum;
- gardenias.
If symptoms of chlorosis are also observed in other plants, regularly spraying the leaves with an iron chelate solution will help maintain a healthy appearance and normal development pets.

Preparation of the drug at home
It is easy to make your own iron chelate at home. When ferrous sulfate is dissolved in water, Fe(II) and Fe(III) ions are formed. A chelating agent (citric acid) traps ferrous iron and supplies it to plants.
The drug is effective for preventive treatments, but contains a large amount of ballast - Fe(III), therefore, when preparing, compliance with the proportions is essential. The working solution must be used immediately while it remains Orange color and transparency.
Cooking method:
- dissolve 5 g in 2 liters of warm distilled or rain water (you can use clean, settled water) citric acid;
- dissolve 8 g of iron sulfate in the same amount of water;
- then slowly pour the vitriol solution into the citric acid solution in a stream, stirring constantly with a wooden stick;
- then pour in another 1 liter of water in the same way and immediately use the solution.
Proportions and sequence must be observed. Treatment must be carried out in the evening or early cloudy (!) morning.
Video instructions for preparing the drug
Iron chelate is used by gardeners when it is necessary to cure plants from iron chlorosis. It is also used when growing crops on poor soil to improve the process of photosynthesis. The fertilizer is sold in stores, it is quickly absorbed by crops and is very effective. At the same time, iron chelate for plants is easy to make at home.
What is Iron Chelate
Iron chelate is an environmentally friendly fertilizer for plants. It is a powdery mass of dirty orange color. It has no taste or smell. Soil usually contains ferric iron. Its molecules are inactive, so it is absorbed by plants in small quantities. Iron chelate contains ferrous iron. It has mobile molecules and is therefore quickly and fully absorbed by crops.
The main problem with divalent iron is that it quickly oxidizes, turning into the trivalent form. To prevent this from happening during use of the drug, iron is placed in a chelate complex (consists of organic acids). Such an element is capable of maintaining a divalent structure for a very long time.
Iron chelate for plants can be used different types, from garden to indoor. After using the drug, it begins to gradually disintegrate, but at the same rate as the absorption of iron by plants, so the crops have time to receive as much of this element as they need.
What is it used for?
Also check out these articles

The range of applications of iron chelate is not extensive. But, in some cases, this is literally an irreplaceable tool. So many gardeners prefer, as they say, to keep it on hand, just in case. So, when is this fertilizer used?
- In the treatment of non-infectious chlorosis, when leaves of crops lose color due to disruption of the photosynthesis process.
- Preventive fertilizing to prevent the development of chlorosis in grapes and other crops with abundant foliage.
- To improve the process of photosynthesis in the leaves of plants that grow in unfavorable conditions or in depleted soil.
Interesting!
Iron is the most abundant element in nature, along with aluminum. But oddly enough, it is its deficiency that plants most often experience.

Before diluting iron chelate, you need to carefully study the instructions and dosages. Otherwise, you can harm the plants by overfeeding them with iron, which is just as dangerous as a lack of this substance. Iron chelate is used in two ways - for root and foliar feeding.
- Foliar feeding is recommended if preventative treatment for plants is needed. The procedure is carried out by spraying the above-ground parts of the crops with a diluted preparation. Used for this special drugs for spraying or spray bottle. For prevention, 2 sprays are enough, and for treatment - 4 treatments with a break of 2-3 weeks. Vegetables, grains, berries, ornamental crops and vineyards should be treated with a 0.4% solution. For fruit trees a 0.8% composition is made.
- Root fertilizer is used if the plant needs to be treated for iron deficiency. A 0.8% solution is used for this. Plants are watered with the mixed liquid strictly at the root, but so that the product does not get on the stem or leaves. One tree consumes 10-20 liters (depending on its age), a bush consumes 1-2 liters, and for processing berries, vegetable plants– 4-5 l/100 sq. m. landing.

Precautionary measures

When using the drug, you must adhere to safety rules. Instructions for use are studied in advance. This product should not be used without personal protection. If the powder or solution gets on the mucous membranes or skin, you should immediately wash it off with water. And if redness, rash or itching appears, you should consult a doctor.
Iron chelate, when diluted, quickly precipitates, losing its beneficial features, therefore it is stored exclusively in dry form.
How to store Iron Chelate
It is advisable to purchase iron chelate in large quantities only for large farms. And yet, even a small package is rarely fully used by gardeners and flower growers, so you need to know how to store the drug. Iron chelate is well stored in dry, undiluted form. The room chosen for it is dry so as not to get damp. Permissible temperature 0…+30 °C. The maximum shelf life is 1.5 years.
The fertilizer should not be exposed to direct sunlight. In addition, the product is kept away from animals, children and birds.

How to make it yourself
You can prepare a solution of iron chelate for plants at home from iron sulfate. This will of course take some time, but such a product will be much cheaper. There are two simple methods.
- A teaspoon of iron sulfate is dissolved in 500 ml of water. Ascorbic acid is added there - 10 g (you can buy it at the pharmacy, but only one that does not contain glucose). Now you need to dilute the resulting mixture with 3 liters of boiled water and mix. That's it, the iron chelate is ready, its concentration is 0.5% on average, so it is quite suitable for spraying.
Interesting!
The breakdown products of iron chelate are water and carbon dioxide. So it does not harm crops, does not accumulate in the ground, and is not hazardous to the environment.
- For 3 liters of boiled water, take a tablespoon of citric acid and a teaspoon of iron sulfate. This mixture must be mixed well. The result is a pale orange liquid, which is used for watering or spraying plants.
Iron chelate prepared at home cannot be stored. It is used after dilution, so it is advisable to make the amount that is necessary for processing the plants.
Iron, as a trace element, is useful and necessary not only for people, but also for plants. With its help, vital processes occur. Including replenishment with macroelements. Hence the need to apply the substance as a fertilizer arises. What happens to a plant if there is not enough iron during development and growth?
The production of chlorophyll is disrupted. This means that photosynthesis stops and the flower or vegetable fruit crop develops slowly and loses the ability to bear fruit. After which he dies. Iron chelate is a fertilizer mixture that will help restore the necessary processes for life.
Composition and chemical formula of the drug
A microelement such as iron is contained in the soil and there is quite a lot of it. Unfortunately, the substance in the soil is of such a form that it is not completely absorbed by crops. Therefore, iron chelate:
- promotes recovery;
- provides a sufficient amount of useful elements;
- allows all cultivated plants to actively develop.
The proposed drug has different composition: from one to several ingredients. The formula includes free atoms of neutral organic matter. It is considered a highly effective microfertilizer.
Due to the presence of a shell in which microgranules are located, the incoming elements are released only after the latter disintegrates. Then recharge occurs.
Iron chelate and its formula consists of ferrous iron. In the soil there is 3-valent. Lower valence allows it to be absorbed in a short time. But there are also disadvantages - the introduced element can also quickly turn into a 3-valent substance (rust).
To prevent such a transformation of iron, a chelate shell was created. The combination of the shell and ferrous iron creates a symbiosis that is ideal for absorbing the substance into the juices of the plant.
Benefits of using the product
Ferovit iron chelate has been and remains the main supplier of the necessary trace element. But most beginning farmers and flower growers have an interesting question. To which they want to receive an answer: “How beneficial are such applications for plants (especially flowers)?” Let's study the main advantages of using iron chelate:
- The drug is completely non-poisonous and non-toxic, which definitely has a positive effect on the development of plants and human well-being.
- Suitable for foliar feeding. Although you can fertilize in other ways.
- Combination with other fertilizers is allowed. This compound has the potential of complex fertilizers. They bring 2 times more benefits. If it is necessary to carry out pest control, iron chelate can be used.
- The substance is applied to the soil in liquid form. The powder dissolves well and quickly.
Read also: Instructions for using Borofoski in the garden
Active absorption through root system occurs only on the third day. The interaction between metal cations and the cells of a flower or other crop begins. This results in transformation into metabolites and enhanced photosynthesis.
The fertilizer is suitable for all crops. But the drug has become most popular for susceptible species:
- raspberries and tomatoes;
- citrus fruits and carrots;
- corn and potatoes;
- corn and grapevines;
- fruit bushes and trees.
In gardening, iron chelate is used extremely rarely, since flowers require less of the trace element.

Scope of application
Iron in chelated form is a sought-after fertilizer. Except large quantity positive qualities, used for various purposes. And not only in the form of foliar feeding of grapes and other vegetable crops.
- small amount of sunlight and ultraviolet radiation. And also their excess;
- low temperatures and temperature changes;
- poor soils.
How to prepare the substance yourself
If urgent application is necessary, there is always the opportunity to prepare iron chelate with your own hands. To prepare, you will need to take: iron sulfate (4 g), citric acid (2.5 g), distilled water or rainwater (1 l).
The components are diluted in different containers. Then slowly stir and pour in. The liquid must be homogeneous. The chelate prepared in this way has a concentration of 0.5 g/l.

There are other recipes that allow you to prepare the drug at home. The cooking technology is the same. But the components are iron sulfate (10 g) and ascorbic acid (20 g).
Need to know! There are many positive reviews about home options. But they cannot be used for a long time. They are designed for single use. After adding a homemade chelate once, be sure to buy a store-bought mixture.
Methods of application
Iron sulfate is non-toxic and fertilizing or other use is allowed at any time during plant development. It’s best to make a schedule or use a gardener’s calendar to regularly feed your green spaces.
Read also: Application of fungicide Skor for the treatment of plants, trees, vineyards, roses

Standard dilution of the powder involves diluting the required dosage into a bucket of water (10 liters). Microfertilizer retains all the necessary elements if diluted in cool water.
Foliar feeding
The use of iron chelate as a foliar feeding (spraying) can be for a preventive purpose, or used as a feeding or therapeutic process. For foliar feeding of a preventive nature, it is carried out no more than 2 times. If the plant is sick, it is recommended to spray 4-5 times.
Mineral fertilizing in the form of spraying is carried out after the first application in 2 or 3 weeks. For fruit trees, iron chelate 0.8% is used. For other crops and grapes – 0.4%.

Root feeding
Feeding raspberries and grapes at the root is carried out as a therapeutic measure. Water solution must be at least 0.8%.
If cucumbers are fertilized during planting in the ground or root fertilizing is required for replanting, then the iron mixture is poured directly under the root system into the prepared holes. The size of the holes should not exceed 25 cm.
Watering is carried out according to the following calculations:
- berry and vegetable crops– per 100 sq. m 5 liters of fertilizer composition;
- for shrubs: for 1 bush 1.5 l;
- for an adult tree – 20 liters (2 buckets), for a young tree – 1 bucket.
Interesting fact! Iron chelate is available in tablets. The instructions differ in the doses for application and the concentration of ingredients. The drug in this form costs less, but is not inferior in effectiveness.
Instructions for use for plants
Chelated iron can be added in two ways. The instructions for use emphasize the time gap between the application of both root and foliar fertilizing.
It is also important to remember the purposes of watering: prevention, treatment of strong or weak chlorosis. The presence of protective clothing is mandatory, regardless of the safety of the substance.
As a preventive measure
It is better to carry out treatment for prevention together with additives (additional components). Suitable: zinc and manganese. Copper will also have a good effect on any crop. The same mixtures develop and restore fruit crops.
For the treatment of chlorosis
Fertilizer for cucumbers and grapes (plant species most susceptible to chlorosis) should include iron. By using a working solution of iron chelate, the symptoms of the disease quickly disappear.
For normal plant life, they require various elements nutrition with which they must be provided throughout the entire time with the only change in the quantity and frequency of application depending on the time of year. One of the most important such elements for plants is iron chelate, which provides reliable protection from manifestations of chlorosis.
Article outline
Features of the drug
The peculiarity of microfertilizer is that it is iron in chelated form. This allows it to be easily and completely absorbed by plants, providing complete nutrition for normal growth. Iron for plants serves as an intermediate element: it is not required as much as macroelements, but not as little as microelements. This makes iron chelate essential for plants.
The lack of this element is easy to notice by appearance, this will be expressed as obvious signs chlorosis. The disease develops against the background of impaired chlorophyll production in the leaves. Its main features are a light leaf blade and characteristic green veins on it.
The appearance of chlorosis occurs when there is an acute deficiency of iron in the soil or as a result of restrictions that have arisen on plants to absorb it in this form. To solve this problem, it is necessary to reconsider agricultural methods, as well as fertilize with iron chelate fertilizer.
Main signs of chlorosis

If at least some of these signs occur, it is necessary to take immediate action and treat chlorosis.
What you need to know about plant chlorosis
Characteristics of microfertilizer
Iron is an active component for plant life; it is actively involved in metabolic processes, and is also one of the components of enzymes that ensure respiration and the formation of chlorophyll.
The main positive qualities of iron chelate
- Completely non-toxic.
- Simultaneous use with mineral fertilizers.
- It dissolves completely in water and is easily absorbed by plants.
- Not exposed to microorganisms.
- It has high transport activity and has high penetration through foliage.
- Simultaneous use with pesticides is possible.
- Versatility in use (used as foliar or root feeding).
An important feature of iron is its availability to plants. There is a misconception that rusty water(which can often be seen from the water tap) is also rich in iron. Yes, it is present there, but in this form it is completely insoluble and sometimes destructive for plants.
There may also be this option: if the soil contains chalk or dolomite, then even the available form of iron under such conditions turns into a completely useless composition for plants. As a result, there will be a lack of this element even if there is sufficient quantity in the soil.
The accessible form of iron can become inaccessible as a result of oxidation, which occurs when interacting with oxygen. After this, it is no longer possible to make the element accessible.
Features of using iron chelate for plants

For plants, of all microelements, it is iron that plays the leading role, since it guarantees the timeliness of chlorophyll biosynthesis, and is also a reliable shield against many diseases, and in particular chlorosis. For plants, the use of iron chelate plays an important role, as it promotes the full development, vegetation and activation of all necessary processes ensuring normal plant growth.
 Regular use of fertilizer guarantees:
Regular use of fertilizer guarantees:
- development of stable immunity of the plant to any negative phenomena external environment;
- compensates for the lack of missing microelements, thereby creating resistance to diseases;
- improves photosynthesis and plant respiration;
- stimulates active growth and full development of plants;
- eliminates iron deficiency;
- normalizes metabolism;
- provides sufficient quantity chlorophyll in leaves.
The use of iron chelate is possible during root tillage, during drip irrigation, and it can also be used as foliar plant nutrition. The maximum effect in cases of obvious signs of iron deficiency in plants can be achieved by foliar treatment of diseased leaves.
It has been noted that among the fruit trees that suffer most from iron deficiency: peach, pear, plum, cherry, apple, citrus trees, and in some cases grapes, provided that they are grown on carbonate or overly limed soils. Signs of the disease can be expressed in poor flowering, weak coloring of fruits, and low tree yields. Also, a deficiency of this element can significantly affect carrots, tomatoes, cucumbers, potatoes, cabbage, corn, and raspberries.
Effect of the drug on indoor plants
 Indoor plants especially need the active element iron, as they have limited space land, in which the emerging shortage of microelements can cause plant diseases and even their death. There are several types of indoor crops that react acutely to a lack of iron in the soil:
Indoor plants especially need the active element iron, as they have limited space land, in which the emerging shortage of microelements can cause plant diseases and even their death. There are several types of indoor crops that react acutely to a lack of iron in the soil:
- gardenias;
- azaleas;
- hydrangeas;
- clerodendrum;
- citrus crops.
Therefore, if there are any crops from the plants listed above in the house, you should know in advance that for their normal functioning they will require regular use of iron chelate for indoor plants. For this purpose, manufacturers produce specially small packaged fertilizers.
Application for indoor plants also includes preventive measures to prevent the occurrence of leaf chlorosis. Indoor crops containing iron in pots are noticeably distinguished by their full and rapid development, and they also develop stable immunity to chlorosis and various stresses. However, if a disease occurs in indoor plants, you can easily cope with it using iron chelate, but do not delay treatment.
How to use microfertilizer
In the treatment of chlorosis and as a preventive measure, iron chelate fertilizer is used in various proportions, time intervals and duration of use.
Instructions for using iron chelate for plants for preventive purposes
It is necessary to dissolve 5 g of the drug in 10 liters of water and spray the foliage generously with the resulting solution, apply the treatment at least 2 times, starting from the first appearance of the leaves and continuing throughout the growing season with an interval of 2 weeks. The last treatment should be carried out before flowering. The consumption rate of the resulting drug is 1 liter per 10 square meters. m.
Instructions for the use of microfertilizer in the treatment of chlorosis
Dissolve 5 g of the substance in 5 liters of water for fruit trees and 8 liters of water for any other crops, then spray the foliage generously and treat at least 4 times every 2 weeks. To enhance treatment, in case of severe chlorosis, root application of the drug can be done; for this purpose, dissolve 5 g of fertilizer per 5 liters of water and the consumption rate of the resulting drug is 2 liters per 1 sq. m. m.
When using the drug, you must follow general rules safety: when processing, wear gloves, a change of clothes, a hat, and also when spraying the drug - safety glasses and a gauze bandage.
- In case of contact with skin or eyes, rinse immediately with plenty of running water.
- After finishing working with the solution, wash your face and hands with soap.
- The fertilizer must be stored in a dry, dark place, out of reach of children. Shelf-life Unlimited. The storage temperature must be at least 0 degrees.
Iron chelate for strawberries - how to use
Making iron chelate at home
In the case when there is no drug at hand, and the plant needs to be saved, you can prepare iron chelate at home, but the resulting solution cannot be stored, so it should be used on the day of preparation.
- Option 1: You will need 1 liter of rainwater, in which you need to dissolve 4 g of citric acid, then add 2.5 g of iron sulfate. Stir the resulting solution until the color of the liquid is uniform. The solution is ready.
- Option 2: You will need 1 liter of rainwater, to which you need to add 10g of iron sulfate and 20g of ascorbic acid, mix everything thoroughly until the ingredients are completely dissolved.
These above solutions can be used for both foliar spraying and root watering. But when using the drug, you must follow all recommendations in order to prevent excess iron in the soil (this is extremely rare).
Signs of excess iron in plants
- the overall growth of the plant stops;
- leaf blades acquire a rich dark green color;
- leaves begin to fall for no apparent reason;
- black necrotic spots appear on the leaf blades;
- The absorption of phosphorus and calcium becomes difficult, so signs of their deficiency appear.
Numerous positive reviews about iron chelate prove that this drug really helps get rid of chlorosis problems, and also helps to develop strong plant immunity to all sorts of negative environmental factors, which guarantees full growth and healthy appearance of leaves.
For normal growth, plants need nutrition rich in useful elements. One of these is iron chelate - an indispensable microfertilizer for plants biologically. active form. Among Ukrainian manufacturers, the leading company in the production of chelate fertilizers and their first developer is SPC "REAKOM".
Features of using iron chelate for plants
There are three possible ways Applications of iron chelate: root tillage, drip irrigation and foliar feeding of plants. Best result gives precisely the foliar method of applying microfertilizers containing iron.When used, iron chelate demonstrates a number of positive qualities:
- Non-toxic.
- Completely soluble in water and easily penetrates foliage.
- Compatible with mineral fertilizers and pesticides.
- Universal in use.
- Elimination of iron deficiency.
- The emergence of immunity to diseases in plants.
- Improving photosynthesis and plant respiration.
- Normalization of metabolism.
- Stimulation of plant growth and development.
- Ensuring sufficient presence of chlorophyll in leaves.
Instructions for use of iron chelate for plants
The proportions, time intervals and duration of use of iron chelate depend on the purpose for which the drug is chosen: preventive or therapeutic.For the purpose of prevention
Feeding plants with iron chelate will not be superfluous if the plant does not have any visible signs of disease.
In this case, it is necessary to measure out 5 g of iron chelate and dissolve it in 10 liters of water. Treatment with the prepared solution is carried out once every 2 weeks by spraying the foliage throughout the growing season. Preventive treatment can begin from the moment the first leaves appear, and should be completed before flowering begins. During this time, spraying should be carried out at least 2 times at a consumption rate of 1 liter per 10 square meters. m.
In the treatment of chlorosis
Chlorosis, caused by a lack of iron, manifests itself in plants in the form of the following symptoms:
- yellowing of the leaf blade with the appearance of green veins;
- reduction of leaves in size;
- delayed development of shoots;
- causeless falling of leaves, flowers and buds;
- deformation of inflorescences;
- curling the sheet along the edges.
If at least one of the listed symptoms of the disease is detected in a plant, it needs treatment. 
For this purpose, it is necessary to dilute 5 g of the drug in 5 liters of water (for fruit trees) and 8 liters of water (for all other types of crops). The foliage is sprayed with the resulting solution once every 2 weeks at least 4 times during the entire cycle. In case of pronounced chlorosis, the effect can be enhanced by root application of iron chelate, for which 5 g of the drug is diluted in 5 liters of water. at the rate of 2 liters per 1 sq. meter.
When working with iron chelate, it is important to follow safety rules: spray with gloves, safety glasses and a gauze bandage. If the solution gets on your skin or eyes, rinse them with water.