British International School
Chemistry abstract
"Inert gases and their properties"
Pupil of 9 b grade
Alexey Sokolenko
Supervisor:
I. V. Chernysheva
I Introduction ………………………………………………………………………… 2
1.1 Inert gases - elements of group VIIIА …………………………………… ... 2
1.2 Argon on Earth and in the Universe ………………………………………………… .5
II History of gas discovery ……………………………………………… .................. 7
2.1 Argon …………………………………………………………………………… 7
2.2 Helium ………………… .. ……………………………………………………… ..8
2.3 Krypton ……………………………………………………… .. ………………… ..9
2.4 Neon ………………………………………………………… .. ………………… 9
2.5 Xenon ……………………………………………………………. …………… .9
2.6 Radon …………………………………………………………… .. …………… .10
III Properties of inert gases and their compounds …………………………………………………………………………………… ..... 10
3.1 Physical properties of inert gases ………………………………………… .10
3.2 Chemical properties of inert gases ……………………………………… ..... 11
3.3 Getting Argon ………………………………………………… ... ………… ..14
3.4 Physiological properties of inert gases …………………………………… 15
IV Use of inert gases ……………………………………………… ..… ..16
List of used literature …………………………………………… .... 18
I Introduction.
Everywhere and everywhere we are surrounded by atmospheric air. What does it consist of? The answer is not difficult: of 78.08 percent nitrogen, 20.9 percent oxygen, 0.03 percent carbon dioxide, 0.00005 percent hydrogen, about 0.94 percent falls on the so-called inert gases. The latter were discovered only at the end of the last century.
Radon is formed during the radioactive decay of radium and is found in trace amounts in uranium-containing materials, as well as in some natural waters. Helium, which is a product of radioactive α-decay of elements, is sometimes found in significant quantities in natural gas and gas emitted from oil wells. This element is found in huge quantities in the Sun and other stars. It is the second most abundant element in the universe (after hydrogen).
1.1 Inert gases - elements of group 8A.
Configuration of the outer electron layer of helium atoms 1 s 2, other elements of subgroup VIII - ns 2 np 6 .

1.2 Argon on earth and in the universe.
There is much more argon on Earth than all the other elements of its group put together. Its average content in the earth's crust (clarke) is 14 times more than helium and 57 times more than neon. There is argon in water, up to 0.3 cm 3 in a liter of sea water and up to 0.55 cm 3 in a liter of fresh water. It is curious that more argon is found in the air of the swim bladder than in the atmospheric air. This is because argon is better soluble in water than nitrogen ... The atmosphere is the main "storage" of earth's argon. It contains (by weight) 1.286%, and 99.6% of atmospheric argon is the heaviest isotope - argon-40. The fraction of this isotope is even greater in argon in the earth's crust. Meanwhile, for the overwhelming majority of light elements, the picture is the opposite - light isotopes predominate. The reason for this anomaly was discovered in 1943. The earth's crust contains a powerful source of argon-40 - the radioactive isotope of potassium 40 K. At first glance, there is not much of this isotope in the bowels - only 0.0119% of the total potassium content. However, the absolute amount of potassium-40 is high, since potassium is one of the most abundant elements on our planet. Each ton of igneous rocks contains 3.1 g of potassium-40. The radioactive decay of potassium-40 atomic nuclei proceeds simultaneously in two ways. Approximately 88% of potassium-40 undergoes beta decay and is converted to calcium-40. But in 12 cases out of 100 (on average) potassium-40 nuclei do not emit, but, on the contrary, capture one electron each from the K-orbit closest to the nucleus ("K-capture"). The captured electron combines with a proton - a new neutron is formed in the nucleus and a neutrino is emitted. The atomic number of the element decreases by one, while the mass of the nucleus remains practically unchanged. This is how potassium is converted to argon. The half-life of 40 K is quite long - 1.3 billion years. Therefore, the formation of 40 Ar in the bowels of the Earth will continue for a long, very long time. Therefore, although extremely slowly, the argon content in the earth's crust and atmosphere will grow steadily, where argon is “exhaled” by the lithosphere as a result of volcanic processes, weathering and recrystallization of rocks, as well as water sources. True, during the existence of the Earth, the supply of radioactive potassium has been thoroughly depleted - it has become 10 times less (if the age of the Earth is assumed to be 4.5 billion years). The isotope ratio 40 Ar: 40 K and 40 Ar: 36 Ar in rocks formed the basis of the argon method for determining the absolute age of minerals. Obviously, the larger this relationship, the older the breed. The argon method is considered to be the most reliable method for determining the age of igneous rocks and most potash minerals. For the development of this method, Professor E.K. Gerling was awarded the Lenin Prize in 1963. So, all or almost all of argon-40 originated on Earth from potassium-40. Therefore, the heavy isotope dominates in terrestrial argon. This factor explains, by the way, one of the anomalies of the periodic system. Contrary to the original principle of its construction - the principle of atomic weights - argon is placed in the table ahead of potassium. If light isotopes predominated in argon, as in neighboring elements (as it seems to be the case in space), then the atomic weight of argon would be two or three units less ... Now about light isotopes. Where do 36 Ar and 38 Ar come from? It is possible that some of these atoms are of relic origin, i.e. part of light argon entered the earth's atmosphere from space during the formation of our planet and its atmosphere. But most of the light isotopes of argon were born on Earth as a result of nuclear processes. Probably, not all such processes have been discovered yet. Most likely, some of them stopped long ago, since the short-lived atoms - "parents" have been exhausted, but there are still ongoing nuclear processes in which argon-36 and argon-38 are born. This is the beta decay of chlorine-36, shelling with alpha particles (in uranium minerals) sulfur-33 and chlorine-35:
36 17 Cl β - → 36 18 Ar + 0 –1 e + ν.
33 16 S + 4 2 He → 36 18 Ar + 1 0 n .
35 17 Cl + 4 2 He → 38 18 Ar + 1 0 n + 0 +1 e .
In the matter of the Universe, argon is even more abundant than on our planet. It is especially abundant in the matter of hot stars and planetary nebulae. It is estimated that argon in space is greater than chlorine, phosphorus, calcium, potassium - elements that are very common on Earth. Space argon is dominated by isotopes 36 Ar and 38 Ar, argon-40 in the Universe is very small. This is indicated by mass spectral analysis of argon from meteorites. Calculations of the prevalence of potassium are convincing in the same way. It turns out that potassium in space is about 50 thousand times less than argon, while on Earth their ratio is clearly in favor of potassium - 660: 1. And since there is little potassium, then where does argon-40 come from ?!
II History of the discovery of inert gases.
By the end of the 18th century, many of the known gases had been discovered. These included: oxygen, a gas that supports combustion; carbon dioxide - it could be easily detected by a very remarkable property: it muddied lime water; and, finally, nitrogen, which does not support combustion and does not act on lime water. This was the idea of the chemists of that time, the composition of the atmosphere, and someone other than the famous English scientist Lord Cavendish did not doubt it.
And he had reason to doubt.
In 1785, he did a fairly simple experiment. First of all, he removed carbon dioxide from the air. He acted on the remaining mixture of nitrogen and oxygen with an electric spark. Nitrogen, reacting with oxygen, gave violent vapors of nitrogen oxides, which, dissolving in water, turned into nitric acid. This operation was repeated many times.
However, slightly less than one hundredth of the volume of air taken for the experiment remained unchanged. Unfortunately, this episode was not forgotten for many years.
In 1785, the English chemist and physicist G. Cavendish discovered some new gas in the air that was unusually stable chemically. This gas accounted for about one hundred and twentieth of the volume of air. But what kind of gas it was, Cavendish was unable to find out. This experience was recalled 107 years later, when John William Stratt (Lord Rayleigh) encountered the same impurity, observing that the nitrogen in the air was heavier than the nitrogen released from the compounds. Not finding a reliable explanation for the anomaly, Rayleigh, through the journal "Nature", turned to his fellow naturalists with a proposal to think together and work on unraveling its causes ... Two years later, Rayleigh and W. Ramsay established that there is indeed an admixture of an unknown gas in the nitrogen of the air, heavier than nitrogen and chemically extremely inert. When they made a public announcement of their discovery, it made a stunning impression. It seemed incredible to many that several generations of scientists who had performed thousands of air analyzes would have overlooked its component, and even such a noticeable - almost a percentage! By the way, it was on this day and hour, August 13, 1894, that argon received its name, which in translation from Greek means "inactive". It was proposed by Dr. Medan, who presided over the meeting. Meanwhile, there is nothing surprising in the fact that argon eluded scientists for so long. Indeed, in nature, he decidedly did not show himself! A parallel with nuclear energy suggests itself: speaking about the difficulties of its identification, A. Einstein noted that it is not easy to recognize a rich man if he does not spend his money ... The skepticism of scientists was quickly dispelled by experimental verification and the establishment of the physical constants of argon. But it was not without moral costs: upset by the attacks of colleagues (mainly chemists), Rayleigh abandoned the study of argon and chemistry in general and focused his interests on physical problems. A great scientist, he also achieved outstanding results in physics, for which in 1904 he was awarded the Nobel Prize. Then in Stockholm, he again met with Ramsay, who on the same day received the Nobel Prize for the discovery and study of noble gases, including argon.
 |
In February 1895, Razmai received a letter from the London meteorologist Myers, where he reported on the experiments of the American geologist Gillebrand, who boiled rare uranium minerals in sulfuric acid and observed the evolution of gas, which resembles nitrogen in its properties. The more uranium is contained in minerals, the more gas is released. Gillebrand tentatively assumed that this gas is nitrogen. "Could it be argon?" - asked the author of the letter.
Soon Razmai sent his assistants to London chemical stores for the uranium mineral kleveite. They bought 30 grams of cleveite, and on the same day Razmai and his assistant Matthews extracted several cubic centimeters of gas. Razmai subjected this gas to a spectroscopic study. He saw a bright yellow line, very similar to the sodium line and at the same time different from it in its position in the spectrum. Razmai was so surprised that he disassembled the spectroscope, cleaned it, but with a new experiment, he again discovered a bright yellow line that did not coincide with the sodium line. Razmai scanned the spectra of all the elements. Finally, he remembered the mysterious line in the spectrum of the solar corona.
 |
In 1868, during a solar eclipse, the French explorer Janssen and the Englishman Lockyer discovered a bright yellow line in the spectrum of solar prominences, which was not found in the terrestrial spectrum of light sources. In 1871, Lockyer suggested whether this line belongs to the spectrum of a substance unknown on Earth.
He called this hypothetical element helium, that is, "solar". But he was not found on the ground. Physicists and chemists were not interested in him: on the Sun, they say, completely different conditions, there and hydrogen will pass for helium.
So is this helium in his hands? Razmai is almost certain of this, but he wants confirmation from renowned spectroscopist Crookes. Razmai sends him a gas for research and writes that he found some new gas, which he called krypton, which means "hidden" in Greek. A telegram from Crookes said, "Krypton is helium."
2.3 Krypton.
 |
By 1895, two inert gases had been discovered. It was clear that there must be another gas between them, the properties of which Razmai described following the example of Mendeleev. Lecoq de Boisbaudran predicted even the weight of the undiscovered gas - 20.0945.
And it is not known whether the scientist would have discovered new inert gases if, during his searches, Linde in Genmania and Hempson in England did not simultaneously take a patent for a machine that liquefies air.
This machine seems to have been specially designed to detect inert gases. The principle of its operation is based on a well-known physical phenomenon, if you compress air, then let it expand quickly, it cools. The cooled air is used to cool a new portion of air entering the machine, etc., until the air turns into liquid.
Having evaporated almost all the nitrogen and oxygen, Mash the remaining liquid air placed in the gasometer. He thought to find helium in it, since he believed that this gas evaporates more slowly than oxygen and nitrogen. He cleaned the gas in the gas meter from the admixture of oxygen and nitrogen and took a spectrum, in which he removed two previously unknown lines.
Next, Razmai placed 15 liters of argon in a cylinder in liquid air. In order to find an inert gas, according to calculations, lighter than argon and krypton, Razmai collected the first portions of argon evaporation. The result is a new spectrum with bright red lines. Razmai called the allocated gas neon, which in Greek means “new”.
Next, Razmai placed 15 liters of argon in a cylinder in liquid air. In order to find an inert gas, according to calculations, lighter than argon and krypton, Razmai collected the first portions of argon evaporation. The result is a new spectrum with bright red lines. Razmai called the new gas neon, which in Greek means “new”.
2.5 Xenon.
In 1888, Razmaya's assistant Travers built a machine capable of producing a temperature of -253 0 C. Solid argon was obtained with it. All gases were driven off except for krypton. And already in the untreated krypton, xenon ("alien") was found. In order to obtain 300 cubic centimeters of xenon, scientists had to process 77.5 million liters of atmospheric air within 2 years.
It has already been said that helium is present in uranium minerals. The more uranium in the slab, the more helium. Razmai tried for a long time to find a relationship between the content of uranium and helium, but he failed. The answer came from the other side; it was associated with the discovery of radioactivity.
They found that radium gives off a gaseous substance called emanation. 1 gram of radium per day gave off one cubic millimeter of emanation. In 1903, Razmai and the famous physicist Soddy took up the study of emanation. They had only 50 milligrams of radium bromide at their disposal; at the same time they had no more than 0.1 cubic millimeter of emanation.
To carry out the work, Razmai built a supersensitive balance that reads four billionths of a gram. Researchers soon discovered that emanation is the last member of the noble gas family.
For a long time they did not manage to consider the spectrum of emanation. Once, leaving the tube with emanation for several days, they put it into the spectroscope and were surprised to see the well-known helium lines in the spectroscope.
This fact confirmed the assumption of Rutherford and Soddy that radioactive transformation is associated with the transformation of atoms. Radium, spontaneously decayed, turned into emanation and gave off the nucleus of the helium atom. One element turned into another.
Scientists have understood why helium is found in uranium materials; he is one of the decay products of uranium. In 1923, by decision of the International Committee on Chemical Elements, emanation was renamed radon.
III Properties of inert gases and their compounds.
3.1 Physical properties of inert gases.
Noble gases are colorless and odorless monoatomic gases.
Inert gases have a higher electrical conductivity compared to other gases and when current passes through them they shine brightly: helium has a bright yellow light, because in its relatively simple spectrum the double yellow line prevails over all others; neon has a fiery red light, since its brightest lines lie in the red part of the spectrum.
The saturated character of atomic molecules of inert gases is also reflected in the fact that inert gases have lower liquefaction and freezing points than other gases with the same molecular weight. Of the subgroup of heavy inert gases, argon is the lightest. It is 1.38 times heavier than air. It becomes liquid at -185.9 ° C, solidifies at -189.4 ° C (under normal pressure).

Unlike helium and neon, it adsorbs quite well on the surfaces of solids and dissolves in water (3.29 cm 3 in 100 g of water at 20 ° C). Argon dissolves even better in many organic liquids. But it is practically insoluble in metals and does not diffuse through them.
3.2 Chemical properties of inert gases.
For a long time, no conditions were found under which noble gases could enter into chemical interaction. They did not form true chemical compounds. In other words, their valence was zero. On this basis, it was decided to consider the new group of chemical elements zero. The low chemical activity of noble gases is explained by the rigid eight-electron configuration of the outer electron layer. The polarizability of atoms increases with the number of electron layers. Consequently, it should increase when going from helium to radon. The reactivity of noble gases should also increase in the same direction.



So, already in 1924, the idea was expressed that some compounds of heavy inert gases (in particular, fluorides and xenon chlorides) are thermodynamically quite stable and can exist under normal conditions. Nine years later, this idea was supported and developed by famous theorists - Pauling and Oddo. The study of the electronic structure of the shells of krypton and xenon from the standpoint of quantum mechanics led to the conclusion that these gases are able to form stable compounds with fluorine. There were also experimenters who decided to test the hypothesis, but time passed, experiments were carried out, and xenon fluoride was not obtained. As a result, almost all work in this area was stopped, and the opinion about the absolute inertness of noble gases was finally confirmed.
However, in 1961, Bartlett, an employee of one of the universities in Canada, studying the properties of platinum hexafluoride, a compound more active than fluorine itself, found that the ionization potential of xenon is lower than that of oxygen (12, 13 and 12, 20 eV, respectively). Meanwhile, oxygen formed with platinum hexafluoride a compound of the composition O 2 PtF 6 ... Bartlett sets up an experiment and at room temperature from gaseous platinum hexafluoride and gaseous xenon obtains a solid orange-yellow substance - xenon hexafluoroplatinate XePtF 6, the behavior of which is no different from the behavior of ordinary chemical compounds. When heated in vacuum, XePtF 6 sublimes without decomposition, hydrolyzes in water, releasing xenon:
2XePtF 6 + 6H 2 O = 2Xe + O 2 + 2PtO 2 + 12HF
Bartlett's subsequent work made it possible to establish that xenon, depending on the reaction conditions, forms two compounds with platinum hexafluoride: XePtF 6 and Xe (PtF 6) 2; when hydrolyzed, the same end products are obtained. Convinced that xenon had indeed reacted with platinum hexafluoride, Bartlett made a presentation and in 1962 published an article in the journal Proceedings of the Chemical Society on his discovery. The article aroused great interest, although many chemists reacted to it with undisguised disbelief. But three weeks later, Bartlett's experiment was repeated by a group of American researchers led by Chernik at the Argonne National Laboratory. In addition, they were the first to synthesize analogous xenon compounds with ruthenium, rhodium and plutonium hexafluorides. This is how the first five xenon compounds were discovered: XePtF 6, Xe (PtF 6) 2, XeRuF 6, XeRhF 6, XePuF 6 - the myth of the absolute inertness of noble gases was dispelled and the beginning of xenon chemistry was laid. It is time to test the correctness of the hypothesis about the possibility of direct interaction of xenon with fluorine.
A mixture of gases (1 part xenon and 5 parts fluorine) was placed in a nickel (since nickel is the most resistant to fluorine) vessel and heated under a relatively low pressure. After an hour, the vessel was quickly cooled, and the remaining gas was evacuated and analyzed. It was fluoride. All xenon reacted! The vessel was opened and found in it colorless crystals of XeF 4. Xenon tetrafluoride turned out to be quite a stable compound, its molecule has the shape of a square with fluorine ions in the corners and xenon in the center. Xenon tetrafluoride fluorinates mercury:
XeF 4 + 2Hg = Xe + 2HgF 2
Platinum is also fluorinated by this substance, but only dissolved in hydrogen fluoride.
It is interesting in the chemistry of xenon that, by changing the reaction conditions, one can obtain not only XeF 4, but also other fluorides — XeF 2, XeF 6.
Soviet chemists V.M.Khutoretsky and V.A. According to the method they proposed, a mixture of xenon and fluorine (in a molecular ratio of 1: 1) is fed into a vessel made of nickel or stainless steel, and when the pressure rises to 35 atm, a spontaneous reaction begins.
XeF 2 is the only xenon fluoride that can be obtained without using elemental fluorine. It is formed by the action of an electric discharge on a mixture of xenon and carbon tetrafluoride. Direct synthesis is, of course, also possible. Very pure XeF 2 is obtained if a mixture of xenon and fluorine is irradiated with ultraviolet light. The solubility of difluoride in water is low, but its solution is the strongest oxidizing agent. Gradually, it self-decomposes into xenon, oxygen and hydrogen fluoride; decomposition is especially rapid in an alkaline environment. Difluoride has a pungent specific odor. Of great theoretical interest is a method for the synthesis of xenon difluoride, based on the action of ultraviolet radiation on a gas mixture (wavelength of the order of 2500-3500 A). Radiation causes the splitting of fluorine molecules into free atoms. This is the reason for the formation of difluoride: atomic fluorine is unusually active. To obtain XeF 6, more stringent conditions are required: 700 ° C and 200 atm. Under these conditions, in a mixture of xenon and fluorine (ratio from 1: 4 to 1:20), almost all of the xenon is converted into XeF 6. Xenon hexafluoride is extremely reactive and explosively decomposes. It readily react with alkali metal fluorides (except LiF):
XeF 6 + RbF = RbXeF 7,
but at 50 ° C this salt decomposes:
2RbXeF 7 = XeF 6 + Rb 2 XeF 8
It was also reported about the synthesis of higher fluoride XeF 8, which is stable only at temperatures below minus 196 ° C.
The synthesis of the first xenon compounds raised the question of the place of inert gases in the periodic table for chemists. Previously, noble gases were separated into a separate zero group, which fully corresponded to the idea of their valence. But when xenon entered into a chemical reaction, when its higher fluoride became known, in which the valency of xenon is eight (and this is quite consistent with the structure of its electron shell), it was decided to transfer the inert gases to group VIII. The zero group ceased to exist.
It has not yet been possible to force xenon to react without the participation of fluorine (or some of its compounds). All currently known xenon compounds are obtained from its fluorides. These substances are highly reactive. The best studied is the interaction of xenon fluorides with water. Hydrolysis of XeF 4 in an acidic medium leads to the formation of xenon oxide XeO 3 - colorless crystals spreading in the air. The XeO 3 molecule has the structure of a flattened triangular pyramid with a xenon atom at the top. This connection is extremely unstable; during its decomposition, the power of the explosion approaches the power of the explosion of TNT. A few hundred milligrams of XeO 3 is enough to blow the desiccator into pieces. It is possible that over time, xenon trioxide will be used as a crushing explosive. Such explosives would be very convenient, because all products of an explosive reaction are gases. In the meantime, it is too expensive to use xenon trioxide for this purpose - after all, xenon in the atmosphere is less than gold in seawater, and the process of its extraction is too laborious. Let us recall that to obtain 1 m 3 of xenon, it is necessary to process 11 million m 3 of air. The corresponding unstable acid trioxide of hexavalent xenon H 6 XeO 6 is formed as a result of the hydrolysis of XeF 6 at 0 ° C:
XeF 6 + 6H 2 О = 6HF + H 6 XeO 6
If Ba (OH) 2 is quickly added to the products of this reaction, a white amorphous precipitate Ba 3 XeO 6 forms. At 125 ° C, it decomposes into barium oxide, xenon and oxygen. Similar sodium and potassium xenonate salts were obtained. Under the action of ozone on a solution of XeO 3 in 1 molar sodium hydroxide, a salt of the higher acid xenon Na 4 XeO 6 is formed. Sodium perxenonate can be isolated in the form of a colorless crystalline hydrate Na4XeO6 · 6H 2 O. The hydrolysis of XeF 6 in sodium and potassium hydroxides also leads to the formation of perxenonates. If the solid salt Na 4 XeO 6 is treated with a solution of lead, silver or uranyl nitrate, the corresponding perxenonates are obtained: PbXeO 6 and (UO 2) 2XeO 6 yellow and Ag 4 XeO 6 black. Similar salts give potassium, lithium, cesium, calcium.
The oxide corresponding to the higher xenon acid is obtained by reacting Na 4 XeO 6 with anhydrous cooled sulfuric acid. This is xenon tetroxide XeO 4. In it, as in octafluoride, the valency of xenon is eight. Solid tetroxide at temperatures above 0 ° C decomposes into xenon and oxygen, and gaseous (at room temperature) into xenon trioxide, xenon and oxygen. The XeO 4 molecule has the shape of a tetrahedron with a xenon atom in the center. Depending on the conditions, the hydrolysis of xenon hexafluoride can proceed in two ways; in one case, tetraoxyfluoride XeOF 4 is obtained, in the other - dioxyfluoride XeO 2 F 2. Direct synthesis from the elements leads to the formation of XeOF 2 oxyfluoride. These are all colorless solids that are stable under normal conditions.
The recently studied reaction of xenon difluoride with anhydrous HClO 4 is very interesting. As a result of this reaction, a new xenon compound XeClO 4 was obtained - an extremely powerful oxidizing agent, probably the strongest of all perchlorates.
Oxygen-free xenon compounds have also been synthesized. Mostly these are double salts, the products of the interaction of xenon fluorides with antimony, arsenic, boron, tantalum fluorides: XeF 2 · SbF 5, XeF 6 · AsF 3, XeF 6 · BF 3 and XeF 2 · 2TaF 5. Finally, substances of the XeSbF 6 type, which are stable at room temperature, and XeSiF 6, an unstable complex, have been obtained.
At the disposal of chemists there are very small amounts of radon, however, it was possible to establish that it also interacts with fluorine, forming non-volatile fluorides. For krypton, difluoride KrF2 and tetrafluoride KrF 4 have been isolated and studied by properties reminiscent of xenon compounds.
3.3 Getting Argon.
The Earth's atmosphere contains 66 · 10 13 tons of argon. This source of argon is inexhaustible, especially since almost all argon sooner or later returns to the atmosphere, since it does not undergo any physical or chemical changes during use. The exception is very small amounts of argon isotopes spent on obtaining new elements and isotopes in nuclear reactions. Argon is obtained as a by-product when air is separated into oxygen and nitrogen. Typically, air separation devices for double rectification are used, consisting of a lower high pressure column (preliminary separation), an upper low pressure column and an intermediate condenser-evaporator. Ultimately, nitrogen is removed from the top and oxygen from the space above the condenser. The volatility of argon is greater than that of oxygen, but less than that of nitrogen. Therefore, the argon fraction is taken at a point located approximately one third of the height of the upper column and taken to a special column. The composition of the argon fraction: 10 ... 12% argon, up to 0.5% nitrogen, the rest is oxygen. In an "argon" column connected to the main apparatus, argon is obtained with an admixture of 3 ... 10% oxygen and 3 ... 5% nitrogen. This is followed by the purification of "raw" argon from oxygen (chemically or by adsorption) and from nitrogen (rectification). Argon is produced on an industrial scale up to 99.99% purity. Argon is also recovered from the wastes of the ammonia production - from the nitrogen left after most of it has been bound with hydrogen. Argon is stored and transported in 40 liter cylinders, painted gray with a green stripe and green inscription. The pressure in them is 150 atm. Transportation of liquefied argon is more economical, for which Dewar vessels and special tanks are used. Artificial radioisotopes of argon were obtained by irradiating some stable and radioactive isotopes (37 Cl, 36 Ar, 40 Ar, 40 Ca) with protons and deuterons, as well as by irradiating with neutrons the products formed in nuclear reactors during the decay of uranium. Isotopes 37 Ar and 41 Ar are used as radioactive indicators: the first is in medicine and pharmacology, the second is in the study of gas flows, efficiency of ventilation and in various scientific research. But, of course, these are not the most important uses of argon.
3.4Physiological action of inert gases.
It was natural to expect that such chemically inert substances as inert gases should not affect living organisms either. But this is not the case. Inhalation of higher inert gases (of course, mixed with oxygen) brings a person into a state similar to alcohol intoxication. The narcotic effect of inert gases is caused by dissolution in nerve tissues. The higher the atomic weight of an inert gas, the greater its solubility and the stronger its narcotic effect.
Now about the effect of argon on a living organism. When a mixture of 69% Ar, 11% nitrogen and 20% oxygen is inhaled under a pressure of 4 atm, anesthesia phenomena occur, which are much more pronounced than when air is inhaled under the same pressure. The anesthesia disappears instantly after the argon supply is stopped. The reason is the non-polarity of argon molecules, while the increased pressure increases the solubility of argon in nerve tissues. Biologists have found that argon is beneficial to plant growth. Even in an atmosphere of pure argon, the seeds of rice, corn, cucumber and rye threw out sprouts. Onions, carrots and lettuce germinate well in an atmosphere of 98% argon and only 2% oxygen.
IV Use of inert gases.
Helium is an important source of low temperatures. At the temperature of liquid helium, the thermal motion of atoms and free electrons in solids is practically absent, which makes it possible to study many new phenomena, for example, superconductivity in the solid state.
Helium gas is used as a light gas to fill balloons. Since it is non-flammable, it is added to hydrogen to fill the airship shell.


Since helium is less soluble in blood than nitrogen, large amounts of helium are used in breathing mixtures for work under pressure, for example, during sea diving, when creating underwater tunnels and structures. When helium is used, decompression (release of dissolved gas from the blood) in a diver is less painful, decompression sickness is less likely, the phenomenon of nitrogen anesthesia is excluded, which is a constant and dangerous companion of the diver's work. Due to their low viscosity, He – O 2 mixtures are used to relieve asthma attacks and various diseases of the respiratory tract.
Helium is used as an inert medium for arc welding, especially magnesium and its alloys, in the production of Si, Ge, Ti and Zr, for cooling nuclear reactors.
Other uses of helium are for gas lubrication of bearings, neutron counters (helium-3), gas thermometers, X-ray spectroscopy, food storage, high voltage switches. Mixed with other noble gases, helium is used in outdoor neon advertising (gas discharge tubes). Liquid helium is beneficial for cooling magnetic superconductors, particle accelerators, and other devices. An unusual application of helium as a refrigerant is the process of continuously mixing 3 He and 4 He to create and maintain temperatures below 0.005 K
The applications for xenon are varied and sometimes unexpected. Man uses both his inertness and his miraculous ability to react with fluorine. High pressure xenon lamps are recognized in lighting engineering. In such lamps, an arc discharge shines in xenon, which is under a pressure of several tens of atmospheres. The light in xenon lamps appears immediately after switching on, it is bright and has a continuous spectrum - from ultraviolet to near infrared. Xenon is also used by doctors - for fluoroscopic examinations of the brain. Like the barite porridge used to scan the intestines, xenon strongly absorbs X-rays and helps to find lesions. Moreover, it is completely harmless. The active isotope of element No. 54, xenon - 133, is used in the study of the functional activity of the lungs and heart.
By blowing argon through liquid steel, gas inclusions are removed from it. This improves the properties of the metal.
Electric arc welding in argon is increasingly used. The argon jet can weld thin-walled products and metals that were previously considered difficult to weld. It is no exaggeration to say that the electric arc in an argon atmosphere revolutionized the technique of cutting metals. The process has accelerated much, it became possible to cut thick sheets of the most refractory metals. Argon blown along the arc column (mixed with hydrogen) protects the cut edges and the tungsten electrode from the formation of oxide, nitride and other films. At the same time, it compresses and concentrates the arc on a small surface, which causes the temperature in the cutting zone to reach 4000-6000 ° C. In addition, this gas jet blows out the cut products. When welding in an argon stream, there is no need for fluxes and electrode coatings, and therefore, in cleaning the seam from slag and flux residues.
Neon and argon are used as fillers in neon and fluorescent lamps. Krypton is used to fill ordinary lamps in order to reduce evaporation and increase the brightness of the tungsten filament. Xenon is filled with high-pressure quartz lamps, which are the most powerful light sources. Helium and argon are used in gas lasers.

List of used literature
1. Petrov M.M., Mikhilev L.A., Kukushkin Yu.N. "Inorganic chemistry"
2. Guzey L.S. Lectures on General Chemistry "
3. Akhmetov NS "General and inorganic chemistry"
4. Nekrasov B.V. "Textbook of General Chemistry"
5. Glinka N.L. "General chemistry
6. Khodakov Yu.V. "General and inorganic chemistry"
In this article, we will focus on VIIIA-group.
These are the elements: helium(He), neon(Ne), argon(Ar), krypton(Kr), xenon(Xe) (these are basic) as well as radioactive radon(Rn).
And formally, artificially obtained ununoctium (Uuo) can also be referred to here.
This group of elements also has its own name - aerogens, but more often they are called noble, or inert gases.
Inert gases
These gases are united by low reactivity. The word inertia is precisely what is meant by inactivity. Therefore, they did not even know about their existence for a long time. You cannot define them using reactions. They were discovered in the air (hence the name aerogens), removing oxygen and other "by-gases" from it to obtain nitrogen, and it was experimentally established that the nitrogen thus obtained had impurities. These impurities turned out to be inert gases.
To understand what is the reason for the low reactivity of these gases, you need to build their electronic diagrams:

We can see that no unpaired electrons, the orbitals are filled. This is a very favorable state of the electron shell. Therefore, all other elements, forming compounds, tend to acquire the electronic configuration of noble gases (remember the octet rule), because it is energetically beneficial, and atoms, like people, love benefits.
Due to their low activity, the atoms of noble gases do not even combine into diatomic molecules (as they do: O 2, Cl 2, N 2, etc.).
Noble gases exist as monatomic molecules.
It is impossible to say that noble gases are absolutely inert. Some aerogens have free orbitals within the same energy level, which means that the process of electron excitation is possible. At present, in extremely extreme conditions, some compounds of these "lazy" elements from the point of view of chemical activity have been obtained. But in the school curriculum, and even more so in, this is not considered.
Physical properties
- helium and neon are lighter than air, the rest of the noble gases that are below are heavier, due to the increase in atomic mass.
- due to chemical inertness, taste and olfactory receptors cannot detect the presence of noble gases in the air, so they have neither taste nor smell.
Practical significance noble gases.
Helium is a well-known gas for filling balloons, which makes the voice funny. Airships are filled with helium (this gas, unlike hydrogen, is not explosive).
Noble gases are used to create an inert (chemically inactive) atmosphere. Some aerogens are part of breathing mixtures, diluting oxygen with themselves (oxygen is a strong oxidizing agent and cannot be breathed in its pure form).
When a current is passed through the noble gases, they tend to glow brightly. That provides aerogens with application for lighting equipment. Looks pretty spectacular.
Inert gases (noble gases) are elements that form group 18 PS (in the short-period version - the main subgroup of group 8): helium He (atomic number 2), neon Ne (Z = 10), argon Ar (Z = 18) krypton Kr ( Z = 36), xenon Xe (Z = 54) and radon Rn (Z = 86). Inert gases are constantly present in the air (1 m 3 of air contains about 9.4 liters, mainly Ar). Scientists have analyzed the composition of the air since the second half of the 18th century. However, it was not possible to detect inert gases for a long time. Due to their chemical passivity, they did not manifest themselves in ordinary reactions in any way and escaped the field of view of researchers. Only after the discovery of spectral analysis were first discovered helium and argon, and then other inert gases. At the beginning of the 20th century, mankind was surprised to learn that air, so familiar and, it seemed, studied, contains 6 previously unknown elements. 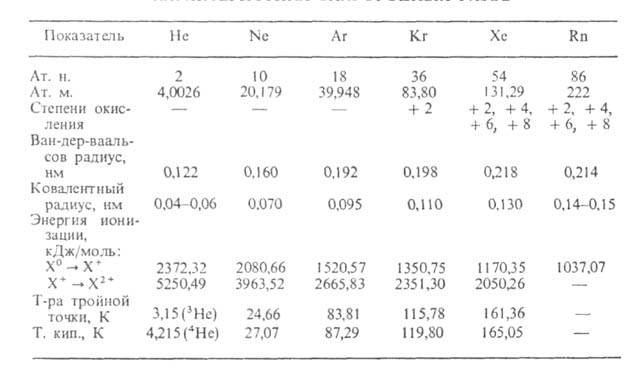
Inert gases are dissolved in water and are found in some rocks. Helium is sometimes found in underground gases. Such gases are its only industrial source. Neon, argon, krypton and xenon are extracted from the air in the process of its separation into nitrogen and oxygen.
The sources of Rn are preparations of uranium, radium, and other radioactive elements. Although all inert gases, except for radon, are stable, their origin is largely due to radioactivity. Thus, helium nuclei, otherwise called ɑ-particles, are constantly formed as a result of the radioactive decay of uranium or thorium. Argon-40, which prevails in the natural mixture of argon isotopes, arises from the radioactive decay of the potassium-40 isotope. Finally, most of the Earth's reserves of Xe are probably due to the spontaneous fission of uranium nuclei.
All inert gases are colorless and odorless. The outer electron shells of their atoms contain the maximum possible number of electrons for the corresponding outer shells: 2 for helium and 8 for the rest. Such casings are highly resistant. This is connected, firstly, with the chemical passivity of inert gases in relation to other elements. And secondly, the inability of their atoms to enter into communication with each other, as a result of which their molecules are monoatomic. Inert gases, especially light gases, are difficult to liquidate. Let's try to figure it out. Why is this so. The molecules of other gases are either permanent dipoles, such as HCl, or easily become dipoles (Cl 2). For permanent dipoles, the "centers of gravity" of positive and negative charges constantly do not coincide with each other. The formation of a dipole in molecules of the Cl 2 type is associated with the displacement of the "centers of gravity" of charges relative to each other under the influence of external forces, in particular, under the action of the electric fields of neighboring molecules. Thus, both in HCl molecules and in Cl 2 molecules between opposite poles of dipoles there are forces of electrostatic attraction. At certain low temperatures, these forces are sufficient to keep the molecules close to each other. At atoms of inert gases, the arrangement of electrons around nuclei is strictly spherical. Therefore, neighboring atoms cannot cause a displacement of the "centers of gravity" of electric charges in their atoms and lead to the formation of an "induced" dipole, as in chlorine molecules. Thus, there are no permanent or induced dipoles in the atoms of inert gases. And if so, then the forces of attraction between them under normal conditions are practically absent. However, due to the constant vibrations of atoms, the "centers" of charges can momentarily shift in different directions of the atom. The forces of electrostatic attraction arising during the formation of this instantaneous dipole are very small, but at very low temperatures they are enough to condense these gases.
For a long time, attempts to obtain conventional chemical compounds of inert gases ended in failure. The Canadian scientist N. Bartlett succeeded in putting an end to the concept of the absolute chemical inactivity of inert gases, who in 1962 reported the synthesis of a xenon compound with platinum hexafluoride PtF 6. The resulting xenon compound had the composition Xe. In subsequent years, a large number of other compounds of radon, xenon and krypton were synthesized.
Let's take a closer look at the chemical properties of inert gases.
Xenon

Due to its low abundance, xenon is much more expensive than the lighter noble gases. To obtain 1 m 3 of xenon, it is necessary to process 10 million m 3 of air. Thus, xenon is the rarest gas in the earth's atmosphere.
By reacting xenon with ice under pressure, its hexahydrate Xe ∙ 6H 2 O was obtained. Under pressure during crystallization of phenol, another clathrate compound with phenol Xe ∙ 6C 6 H 5 OH was isolated. Xenon trioxide XeO 3 in the form of colorless crystals and tetraoxide XeO 4 in the form of gas were obtained and characterized as extremely explosive substances. At 0 ° C, a disproportionation occurs:
2XeO 3 = XeO 4 + Xe + O 2
When interacting with water of xenon tetroxide, where xenon is in the +8 oxidation state, a strong perxenonic acid H 4 XeO 6 is formed, which could not be isolated in an individual state, but salts - alkali metal perxenates were obtained. Only potassium, rubidium and cesium salts were found to be soluble in water.Gaseous xenon reacts with platinum hexafluoride PtF 6 to form xenon hexafluoroplatinate Xe. When heated in a vacuum, it accelerates without decomposition, and in water it hydrolyzes with the release of xenon:
2Xe + 6H 2 O = 2Xe + O 2 + 2PtO 2 + 12HF
Later it turned out that xenon forms 2 compounds with platinum hexafluoride: Xe and Xe 2. When xenon is heated with fluorine, XeF 4 is formed, which fluorinates fluorine and platinum:XeF 4 + 2Hg = Xe + 2HgF 2
XeF 4 + 2Pt = Xe + 2PtF 4
As a result of hydrolysis of XeF 4, unstable XeO 3 is formed, which decomposes in air with an explosion. XeF 2 and XeF b have also been obtained, the latter of which decays with an explosion. It is extremely active, reacts easily with alkali metal fluorides:
XeF 6 + RbF = Rb
The resulting rubidium salt decomposes at 50 ° C to XeF 6 and RbXeF 8With ozone in an alkaline medium, XeO 3 forms the sodium salt Na 4 XeO 6 (sodium perxenonate). The perxenonate anion is the most powerful oxidizing agent known. Xe (ClO-4) 2 is also a strong oxidizing agent. It is the most powerful oxidizing agent of all known perchlorates.
Radon
Radon forms clathrates, which, although they have a constant composition, do not contain chemical bonds with the participation of radon. Known hydrates Rn ∙ 6H 2 O, adducts with alcohols, for example Rn ∙ 2C 2 H 5 OH, etc. With fluorine, radon at high temperatures forms compounds of the composition RnF n, where n = 4, 6, 2.Krypton

Krypton forms clathrate compounds with water, sulfuric acid, halogen, hydrogen, phenol, toulol and other organic substances. When krypton interacts with fluorine, it is possible to obtain its di- and tetrafluorides, which are stable only at low temperatures. Difluoride exhibits oxidizing properties:
KrF 2 + 2HCl = Kr + Cl 2 + 2HF
2KrF 2 + 2H 2 O = 2Kr + O 2 + 4HF
It was not possible to obtain compounds of lighter inert gases. Theoretical calculations have shown that argon compounds may be synthesized, but they cannot be obtained from helium and neon.

In welding, so-called inert gases are often used. These include a group of chemical elements that have similar properties. An inert gas, a noble gas, is monoatomic under normal conditions. Almost all of them are colorless and odorless. Very low chemical reactivity is a characteristic feature. They practically do not react with metals, which is required for normal operation. Such gases occupy the first 6 periods and belong to the eighth group of chemical elements in the periodic table.
The properties of inert gases can be explained by the theory of atomic structures. They get full electron shells from valence electrons. This creates conditions in which a substance can only participate in a small number of chemical reactions. It is worth noting that the differences in boiling and melting points for almost all noble gases are less than 10 degrees Celsius.
Application area
The properties of inert gases make them very popular in the welding field. The main areas of application are gas and gas arc welding. They act as a protective environment that isolates the weld pool with molten metal from the negative effects of various factors, including the air environment. As a rule, they are used together with technical oxygen, as it raises the temperature of their combustion. When using inert gases, the seams are more reliable and of high quality, since the likelihood of defects during work is reduced.
The substances are used on construction sites when joining metal structures, in particular, load-bearing parts. They are more convenient to work with thin parts, pipes and other objects that are difficult to weld electrically. In repair shops for the restoration of cars and other complex equipment, it is inert gas welding that is the main method of joining parts, since it has a delicate attitude to the material. In the public sector, where it comes to repairing pipes and other things, these varieties are also used. In the production of metal products of various types, especially from non-ferrous metals that are difficult to weld, inert noble gas acts as the main raw material for work.
Advantages
Having figured out what an inert gas means, it is worth understanding why it is so popular in this area. This is due to a number of advantages that are based on its properties. Naturally, each of them may have their own characteristics, but in general, the following positive points can be distinguished:
- The substance practically does not react with the metals with which work is carried out, oxygen, the environment, and so on;
- The gases give a sufficiently high temperature during welding, which ensures welding to a great depth of the metal;
- It is possible to regulate the welding flame, its ratio with oxygen to obtain the desired parameters;
- Storage and transportation in a liquefied state or under high pressure turns out to be profitable due to its compactness;
- Extraction of some gases can be carried out directly at the workplace thanks to special installations of generators.
disadvantages
Nevertheless, although gases are one of the most high-quality solutions for this area, their use has certain disadvantages, among which the following are the main ones:
- Storage and transportation of gas cylinders is quite difficult, since there is a risk of explosion;
- Most substances of this kind cause asphyxiation when their concentration in the ambient air reaches a certain value;
- Some of the gases are very harmful to the respiratory system and can cause occupational diseases in a relatively short period of time, therefore, it is imperative to use personal protective equipment;
- Acetylene and other gases of this kind can be expensive, making the welding process more expensive.
Types of inert gases
Argon is non-toxic, odorless and colorless. It is almost 1.5 times heavier than air. Gas does not dissolve in metals, both in solid and liquid state. For industry it is produced in the form of the highest and first grade. The highest grade contains 99.993% pure substance and is used for welding critical joints. The first grade contains 99.98% pure substance. Nitrogen and oxygen are available as additives. Good for.

Helium is non-toxic, odorless and colorless. It is lighter than air. The substance is produced in accordance with GOST 20461-75. It can be industrial gas with a purity of 99.8% and a grade of the highest purity 99.985%. It is not used as often in welding as argon, as it is more expensive and scarce. It is almost 2 times more efficient, since the arc generates more energy with it and provides better protection and deeper penetration. The main field of application is the welding of active and chemically pure materials based on magnesium and aluminum.

Nitrogen is non-toxic, odorless and colorless. It is used for welding copper and alloys from this metal. Produced in accordance with GOST 9293-74 and according to this standard there are 4 main grades. The higher contains 99.9% of pure material, the first contains 99.5%, the second 99%, and the third 97%.
![]()
Instructions for use
When using inert gases, they are first put into the burner to check its functionality. Only then can oxygen be added. The flame can be used for preheating and gradual cooling, not just welding. At the beginning of welding, you need to set the parameters of the ratio of gases and their supply to the desired mode.
Before starting work, you should always check all hoses for integrity so that gas does not escape from them. "
Security measures
- Gas cylinders should be located at a distance of 5 meters from the source of flame and flammable substances;
- There should be no oil stains nearby;
- During operation, the cylinders must be securely fastened;
- It is always necessary to monitor the state of the gas content of the room so that there is no suffocation.
Storage and transportation
Transportation must be carried out in a vehicle with springs. The cylinders must be secured to prevent them from bumping against each other and falling. Storage should be carried out in a ventilated area.
Conclusion
Despite all the disadvantages and difficulties, inert gases remain the most demanded consumables for high-quality and reliable welding.




