Corrosion of steel in steam boilers, occurring under the influence of water vapor, comes down mainly to the following reaction:
3Fe + 4H20 = Fe2O3 + 4H2
We can assume that the inner surface of the boiler represents a thin film of magnetic iron oxide. During operation of the boiler, the oxide film is continuously destroyed and formed again, and hydrogen is released. Since the surface film of magnetic iron oxide represents the main protection for steel, it should be maintained in a state of least permeability to water.
For boilers, fittings, water and steam pipelines, predominantly simple carbon or low-alloy steels are used. The corrosive medium in all cases is water or water vapor of varying degrees of purity.
The temperature at which the corrosion process can occur ranges from the temperature of the room where the inactive boiler is located to the boiling point of saturated solutions when the boiler is operating, sometimes reaching 700°. The solution may have a temperature significantly higher than critical temperature pure water (374°). However, high salt concentrations in boilers are rare.
The mechanism by which physical and chemical causes can lead to film failure in steam boilers is essentially different from the mechanism studied in more low temperatures on less critical equipment. The difference is that the corrosion rate in boilers is much greater due to the high temperature and pressure. The high rate of heat transfer from the boiler walls to the environment, reaching 15 cal/cm2sec, also increases corrosion.
POT CORROSION
The shape of corrosion pits and their distribution on the metal surface can vary widely. Corrosion pits sometimes form within existing pits and are often so close together that the surface becomes extremely uneven.Recognizing pitting corrosion
Determining the cause of corrosion damage certain type often very difficult, since several reasons can act simultaneously; in addition, a number of changes that occur when the boiler cools from high temperature and when water is drained sometimes masks the phenomena that took place during operation. However, experience greatly helps in recognizing pitting corrosion in boilers. For example, it was observed that the presence of black magnetic iron oxide in a corrosion shell or on the surface of a tubercle indicates that an active process was occurring in the boiler. Such observations are often used to check measures taken to protect against corrosion.
The iron oxide that forms in areas of active corrosion should not be mixed with black magnetic iron oxide, which is sometimes present as a suspension in boiler water. It must be remembered that neither total fine magnetic iron oxide, nor the amount of hydrogen released in the boiler can serve as a reliable indicator of the degree and extent of corrosion occurring. Ferrous hydrate entering the boiler from foreign sources, such as condensate tanks or boiler supply piping, may partly explain the presence of both iron oxide and hydrogen in the boiler. Ferrous hydroxide supplied with the feed water reacts in the boiler by reaction.
3Fe (OH)2 = Fe3O4 + 2H2O + H2.
Reasons influencing the development of pitting corrosion
Foreign impurities and stress. Non-metallic inclusions in steel, as well as stress, can create anodic areas on the metal surface. Typically, corrosion pits are different sizes and scattered across the surface in disarray. In the presence of stresses, the location of the shells obeys the direction of the applied stress. Typical examples include fin tubes where fins have cracked, as well as boiler tube flaring areas.
Dissolved oxygen.
It is possible that the most powerful activator of pitting corrosion is oxygen dissolved in water. At all temperatures, even in an alkaline solution, oxygen serves as an active depolarizer. In addition, oxygen concentration elements can easily occur in boilers, especially under scale or contamination, where stagnant areas are created. The usual measure to combat this type of corrosion is deaeration.
Dissolved carbonic anhydride.
Since solutions of carbonic anhydride have a slightly acidic reaction, it accelerates corrosion in boilers. Alkaline boiler water reduces the aggressiveness of dissolved carbonic anhydride; however, the resulting benefit does not extend to steam-washed surfaces or condensate piping. Removal of carbonic anhydride along with dissolved oxygen by mechanical deaeration is common.
Recently, attempts have been made to use cyclohexylamine to eliminate corrosion in steam and condensate lines in heating systems.
Deposits on the walls of the boiler.
Very often, corrosion pits can be found along outer surface(or below the surface) of deposits such as mill scale, boiler sludge, boiler scale, corrosion products, oil films. Once started, pitting corrosion will continue to develop unless the corrosion products are removed. This type of local corrosion is enhanced by the cathodic (in relation to boiler steel) nature of the deposits or by the depletion of oxygen under the deposits.
Copper in boiler water.
If we take into account the large quantities of copper alloys used for auxiliary equipment(condensers, pumps, etc.), then it is not surprising that in most cases boiler deposits contain copper. It is usually present in metallic state, sometimes in the form of an oxide. The amount of copper in deposits varies from fractions of a percent to almost pure copper.
The question of the significance of copper deposits in boiler corrosion cannot be considered resolved. Some argue that copper is only present during the corrosion process and does not affect it in any way; others, on the contrary, believe that copper, being a cathode in relation to steel, can contribute to pitting corrosion. None of these points of view has been confirmed by direct experiments.
In many cases, little (or even no) corrosion was observed despite the deposits throughout the boiler containing significant amounts of copper metal. There is also evidence that when copper comes into contact with mild steel in alkaline boiler water, elevated temperatures, copper deteriorates faster than steel. Copper rings, crimping ends of flared pipes, copper rivets and screens of auxiliary equipment through which boiler water passes are almost completely destroyed even at relatively low temperatures. In view of this, it is believed that copper metal does not increase the corrosion of boiler steel. The deposited copper can be considered simply as the end product of the reduction of copper oxide by hydrogen at the time of its formation.
On the contrary, very strong corrosion pitting of boiler metal is often observed in the vicinity of deposits that are especially rich in copper. These observations led to the suggestion that copper, because it is cathodic to steel, promotes pitting corrosion.
The surface of boilers rarely presents exposed metallic iron. Most often it has protective layer, consisting predominantly of iron oxide. It is possible that where cracks form in this layer, a surface is exposed that is anodic to copper. In such places, the formation of corrosion pits increases. This can also explain, in some cases, accelerated corrosion in those places where a shell has formed, as well as severe pitting corrosion, sometimes observed after cleaning boilers with the use of acids.
Improper maintenance of idle boilers.
One of the most common reasons The formation of corrosion shells is caused by the lack of proper care of idle boilers. An idle boiler must be kept either completely dry or filled with water treated in such a way that corrosion is impossible.
The water remaining on the inner surface of an inactive boiler dissolves oxygen from the air, which leads to the formation of shells, which will later become centers around which the corrosion process will develop.
Common instructions for protecting idle boilers from corrosion are as follows:
1) draining water from a still hot boiler (about 90°); blowing the boiler with air until it is completely dry and kept dry;
2) filling the boiler with alkaline water (pH = 11), containing an excess of SO3 ions (about 0.01%), and storing under a water or steam seal;
3) filling the boiler alkaline solution, containing salts of chromic acid (0.02-0.03% CrO4").
When chemically cleaning boilers, the protective layer of iron oxide will be removed in many places. Subsequently, these places may not be covered with a newly formed continuous layer and shells will appear on them, even in the absence of copper. Therefore, it is recommended immediately after chemical cleaning restore the iron oxide layer by treating with a boiling alkaline solution (similar to what is done for new boilers coming into operation).
Corrosion of economizers
The general provisions regarding boiler corrosion apply equally to economizers. However, the economizer, heating the feed water and located in front of the boiler, is especially sensitive to the formation of corrosion pits. It represents the first high-temperature surface that experiences the destructive action of oxygen dissolved in the feed water. In addition, the water passing through the economizer generally has a low pH value and does not contain chemical retardants.
The fight against corrosion of economizers involves deaerating the water and adding alkali and chemical retarders.
Sometimes boiler water is treated by passing part of it through an economizer. In this case, sludge deposits in the economizer should be avoided. The effect of such boiler water recirculation on steam quality must also be taken into account.
BOILER WATER TREATMENT
When treating boiler water for corrosion protection, the primary objective is to form and maintain a protective film on metal surfaces. The combination of substances added to the water depends on the operating conditions, especially pressure, temperature, thermal tension, and the quality of the feed water. However, in all cases, three rules must be followed: boiler water must be alkaline, must not contain dissolved oxygen and must not pollute the heating surface.Caustic soda provides best protection at pH = 11-12. In practice, with a complex composition of boiler water best results are obtained at pH = 11. For boilers operating at pressures below 17.5 kg/cm2, pH is usually maintained between 11.0 and 11.5. For higher pressures, due to the possibility of metal destruction as a result of improper circulation and a local increase in the concentration of the alkali solution, the pH is usually taken to be 10.5 - 11.0.
To remove residual oxygen, chemical reducing agents are widely used: salts of sulfurous acid, ferrous hydroxide and organic reducing agents. Ferrous compounds are very good at removing oxygen, but form sludge which has an undesirable effect on heat transfer. Organic reducing agents, due to their instability at high temperatures, are usually not recommended for boilers operating at pressures above 35 kg/cm2. There is evidence of the decomposition of sulfuric acid salts at elevated temperatures. However, their use in small concentrations in boilers operating under pressures up to 98 kg/cm2 is widely practiced. Many installations high pressure They operate without chemical deaeration at all.
The cost of special equipment for deaeration, despite its undoubted benefits, is not always justified for small installations operating at relatively low pressures. At pressures below 14 kg/cm2, partial deaeration in feedwater heaters can bring the dissolved oxygen content to approximately 0.00007%. The addition of chemical reducing agents gives good results, especially when the pH of the water is above 11, and oxygen binders are added before the water enters the boiler, which ensures that oxygen is absorbed outside the boiler.
CORROSION IN CONCENTRATED BOILER WATER
Low concentrations of caustic soda (about 0.01%) help maintain the oxide layer on steel in a state that reliably provides protection against corrosion. A local increase in concentration causes severe corrosion.Areas of the boiler surface where the alkali concentration reaches a dangerous value are usually characterized by excessive heat supply in relation to the circulating water. Alkali-enriched zones near the metal surface can appear in different places in the boiler. Corrosion pitting occurs in stripes or elongated areas, sometimes smooth and sometimes filled with hard and dense magnetic oxide.
Tubes located horizontally or slightly inclined and exposed to intense radiation from above are corroded inside, along the upper generatrix. Similar cases were observed in high-power boilers, and were also reproduced in specially designed experiments.
Tubes in which the water circulation is uneven or disrupted due to heavy boiler load may be subject to destruction along the lower generatrix. Sometimes corrosion is more pronounced along the variable water level on the side surfaces. Abundant accumulations of magnetic iron oxide can often be observed—sometimes loose, sometimes forming dense masses.
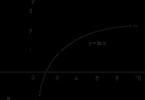
Overheating steel often increases destruction. This can occur as a result of the formation of a layer of steam at the top of the inclined tube. The formation of a steam jacket is also possible in vertical tubes with increased heat supply, as indicated by temperature measurements in various places in the tubes during boiler operation. Typical data obtained from these measurements are presented in Fig. 7. Limited areas of overheating in vertical tubes having a normal temperature above and below the “hot spot” may be the result of film boiling of water.
Every time a steam bubble forms on the surface of the boiler tube, the temperature of the metal underneath rises.
An increase in the concentration of alkali in water should occur at the interface: steam bubble - water - heating surface. In Fig. it has been shown that even a slight increase in the temperature of the water film in contact with the metal and with the expanding steam bubble leads to a concentration of caustic soda, measured in percentages and not parts per million. The film of water enriched with alkali, formed as a result of the appearance of each steam bubble, affects a small area of the metal and for a very short time. However, the total effect of steam on the heating surface can be likened continuous action concentrated alkali solution, despite the fact that total weight water contains only parts per million of caustic soda. Several attempts have been made to find a solution to the issue associated with the local increase in the concentration of caustic soda on heating surfaces. Thus, it was proposed to add neutral salts (for example, metal chlorides) to water in a higher concentration than sodium hydroxide. However, it is best to completely eliminate the addition of caustic soda and ensure the required pH value by introducing hydrolyzable salts of phosphoric acid. The relationship between the pH of the solution and the concentration of sodium phosphorus salt is shown in Fig. Although water containing sodium phosphorus salt has a high pH value, it can be evaporated without significantly increasing the concentration of hydroxyl ions.
It should, however, be remembered that eliminating the action of caustic soda only means that one factor accelerating corrosion has been removed. If a steam jacket forms in the tubes, then even though the water does not contain alkali, corrosion is still possible, although to a lesser extent than in the presence of caustic soda. The solution to the problem should also be sought by changing the design, taking into account at the same time the tendency towards a constant increase in the energy intensity of the heating surfaces, which, in turn, certainly increases corrosion. If the temperature of a thin layer of water directly at the heating surface of the tube exceeds the average temperature of the water in the vessel by at least a small amount, the concentration of caustic soda in such a layer can increase relatively strongly. The curve approximately shows the equilibrium conditions in a solution containing only sodium hydroxide. The exact data depends, to some extent, on the pressure in the boiler.
ALKALINE BRITTLESS OF STEEL
Alkali brittleness can be defined as the appearance of cracks in the area of rivet seams or other joints where concentrated alkali solution may accumulate and where there are high mechanical stresses.The most serious damage almost always occurs in the area of the rivet seams. Sometimes they cause the boiler to explode; More often it is necessary to carry out expensive repairs even on relatively new boilers. One American Railway In one year, 40 locomotive boilers were cracked, requiring repairs costing approximately $60,000. The appearance of brittleness was also observed on tubes in places of flaring, on connections, manifolds and in places of threaded connections.
Stress required to cause alkali embrittlement
Practice shows a low probability of brittle fracture of conventional boiler steel if the stresses do not exceed the yield strength. Stresses created by steam pressure or uniformly distributed load from the structure’s own weight cannot lead to the formation of cracks. However, stresses generated by rolling boiler sheets, deformation during riveting, or any cold working that involves permanent deformation can cause cracks to form.
The presence of externally applied stresses is not necessary for the formation of cracks. A boiler steel sample previously held under constant bending stress and then released may crack in an alkaline solution whose concentration is equal to the increased alkali concentration in the boiler water.
Alkali concentration
The normal concentration of alkali in the boiler drum cannot cause cracks, because it does not exceed 0.1% NaOH, and the lowest concentration at which alkali brittleness is observed is approximately 100 times higher than normal.
Such high concentrations may result from extremely slow percolation of water through a rivet seam or some other gap. This explains the appearance of hard salts on the outside of most rivet seams in steam boilers. The most dangerous leak is the one that is difficult to detect and leaves a residue. solid inside the rivet seam, where there are high residual stresses. The combined action of stress and a concentrated solution can cause the appearance of alkali brittleness cracks.
Alkali embrittlement detection device
A special device for monitoring the composition of water reproduces the process of water evaporation with increasing alkali concentration on a stressed steel sample under the same conditions in which this occurs in the area of the rivet seam. Cracking control sample indicates that boiler water of this composition can cause alkali embrittlement. Therefore, in this case, water treatment is necessary to eliminate its hazardous properties. However, cracking of the control sample does not mean that cracks have already appeared or will appear in the boiler. In rivet seams or other joints there is not necessarily both leakage (steaming), stress, and an increase in alkali concentration, as in the control sample.
The control device is installed directly on the steam boiler and allows you to judge the quality of the boiler water.
The test lasts 30 days or more with constant circulation of water through the control device.
Alkali Brittleness Crack Recognition
Alkali brittleness cracks in conventional boiler steel are of a different nature than fatigue cracks or cracks formed due to high voltage. This is illustrated in Fig. I9, which shows the intergranular nature of such cracks, forming a fine network. The difference between intergranular alkali brittleness cracks and intragranular cracks caused by corrosion fatigue can be seen by comparison.
In alloy steels (for example, nickel or silicon-manganese), used for locomotive boilers, cracks are also arranged in a grid, but do not always pass between crystallites, as in the case of ordinary boiler steel.
Alkali brittleness theory
Atoms in the crystal lattice of a metal located at the boundaries of crystallites experience less symmetrical influence from their neighbors than atoms in the rest of the grain mass. Therefore, they leave the crystal lattice more easily. One might think that with careful selection of an aggressive environment it will be possible to achieve such selective removal of atoms from crystallite boundaries. Indeed, experiments show that in acidic, neutral (with the help of a weak electric current, creating conditions favorable for corrosion) and concentrated alkali solutions, intergranular cracking can be obtained. If the solution causing general corrosion is modified by the addition of some substance that forms a protective film on the surface of the crystallites, the corrosion is concentrated at the boundaries between the crystallites.
The aggressive solution in this case is caustic soda solution. The sodium silica salt can protect the surfaces of crystallites without affecting the boundaries between them. The result of a combined protective and aggressive action depends on many circumstances: concentration, temperature, stressed state of the metal and composition of the solution.
There are also the colloidal theory of alkali brittleness and the theory of the action of hydrogen dissolving in steel.
Ways to combat alkaline embrittlement
One way to combat alkali brittleness is to replace boiler riveting with welding, which eliminates the possibility of leakage. Brittleness can also be eliminated by using steel that is resistant to intergranular corrosion or by chemically treating the boiler water. In riveted boilers currently used, the latter method is the only acceptable one.
Preliminary tests using a control sample represent best way determining the effectiveness of certain protective additives to water. Sodium sulfide salt does not prevent cracking. Sodium nitrogen salt is successfully used to protect against cracking at pressures up to 52.5 kg/cm2. Concentrated sodium nitrogen salt solutions boiling at atmospheric pressure can cause stress corrosion cracks in mild steel.
Currently, sodium nitrogen salt is widely used in stationary boilers. The concentration of sodium nitrogen salt corresponds to 20-30% of the alkali concentration.
CORROSION OF STEAM HEATERS
Corrosion on internal surfaces superheater tubes is caused primarily by the interaction between metal and steam at high temperatures and, to a lesser extent, by the entrainment of boiler water salts by steam. In the latter case, films of solutions with a high concentration of caustic soda can form on the metal walls, directly corroding the steel or producing deposits that sinter on the wall of the tubes, which can lead to the formation of blowouts. In idle boilers and in cases of steam condensation in relatively cold superheaters, pitting corrosion may develop under the influence of oxygen and carbonic anhydride.Hydrogen as a measure of corrosion rate
Steam temperature in modern boilers approaches the temperatures used in industrial production hydrogen by direct reaction between steam and iron.
The rate of corrosion of pipes made of carbon and alloy steel under the influence of steam, at temperatures up to 650°, can be judged by the volume of hydrogen released. Hydrogen evolution is sometimes used as a measure of general corrosion.
IN Lately At power plants in the United States, three types of miniature installations are used to remove gases and air. They ensure complete removal of gases, and the degassed condensate is suitable for determining salts carried away by steam from the boiler. An approximate value of the total corrosion of the superheater during boiler operation can be obtained by determining the difference in hydrogen concentrations in steam samples taken before and after its passage through the superheater.
Corrosion caused by impurities in steam
The saturated steam entering the superheater carries with it small but measurable amounts of gases and salts from the boiler water. The most commonly encountered gases are oxygen, ammonia and carbon dioxide. When steam passes through the superheater, no noticeable change in the concentration of these gases is observed. Only minor corrosion of the metal superheater can be attributed to the action of these gases. It has not yet been proven that salts dissolved in water, dry, or deposited on superheater elements can contribute to corrosion. However, caustic soda, being the main integral part salts carried away by boiler water can contribute to corrosion of a very hot tube, especially if the alkali adheres to the metal wall.
Increasing the purity of saturated steam is achieved by thoroughly removing gases from the feed water. Reducing the amount of salts carried away by steam is achieved by thorough cleaning in the upper collector, using mechanical separators, saturated steam rinsing with feed water or suitable chemical treatment of the water.
Determination of the concentration and nature of gases entrained by saturated steam is carried out using the above devices and chemical analysis. It is convenient to determine the concentration of salts in saturated steam by measuring the electrical conductivity of water or evaporation large quantity condensate
An improved method for measuring electrical conductivity is proposed, and appropriate corrections for some dissolved gases are given. The condensate in the miniature degassing units mentioned above can also be used to measure electrical conductivity.
When the boiler is idle, the superheater is a refrigerator in which condensation accumulates; In this case, normal underwater pitting is possible if the steam contained oxygen or carbon dioxide.
Popular articles
This corrosion is often more significant and dangerous in size and intensity than the corrosion of boilers during operation.
When water is left in systems, depending on its temperature and air access, a wide variety of cases of standstill corrosion can occur. First of all, it should be noted that it is extremely undesirable to have water in the pipes of the units when they are in reserve.
If water for one reason or another remains in the system, then severe static corrosion can be observed in the steam and especially in the water space of the tank (mainly along the waterline) at a water temperature of 60-70°C. Therefore, in practice, stop-time corrosion of varying intensity is often observed, despite the same shutdown modes of the system and the quality of the water contained in them; devices with significant thermal accumulation are subject to more severe corrosion than devices with a firebox size and heating surface, since the boiler water in them cools faster; its temperature becomes below 60-70°C.
At water temperatures above 85-90°C (for example, during short-term shutdowns of apparatus), overall corrosion decreases, and the corrosion of the metal of the steam space, in which increased condensation of vapors is observed in this case, may exceed the corrosion of the metal of the water space. Standstill corrosion in the steam space is in all cases more uniform than in the water space of the boiler.
The development of standstill corrosion is greatly facilitated by sludge accumulating on the surfaces of the boiler, which usually retains moisture. In this regard, significant corrosion pits are often found in units and pipes along the lower generatrix and at their ends, i.e. in areas largest accumulation sludge.
Methods for preserving equipment in reserve
The following methods can be used to preserve equipment:
a) drying - removing water and moisture from aggregates;
b) filling them with solutions of caustic soda, phosphate, silicate, sodium nitrite, hydrazine;
c) filling technological system nitrogen.
The preservation method should be selected depending on the nature and duration of the downtime, as well as the type and design features of the equipment.
Equipment downtime can be divided into two groups based on duration: short-term—no more than 3 days and long-term—more than 3 days.
There are two types of short-term downtime:
a) planned, related to being put into reserve on weekends due to a drop in load or put into reserve at night;
b) forced - due to failure of pipes or damage to other equipment components, the elimination of which does not require a longer shutdown.
Depending on the purpose long downtimes can be divided into the following groups: a) putting equipment into reserve; b) current repairs; c) major repairs.
During short-term equipment downtime, it is necessary to use preservation by filling with deaerated water and maintaining overpressure or gas (nitrogen) method. If emergency shutdown is necessary, nitrogen preservation is the only acceptable method.
When the system is put on standby or is idle for a long time without executing repair work It is advisable to preserve it by filling it with a solution of nitrite or sodium silicate. In these cases, nitrogen conservation can also be used, making sure to take measures to create system density in order to prevent excessive gas consumption and unproductive operation of the nitrogen installation, as well as create safe conditions when servicing equipment.
Preservation methods by creating excess pressure and filling with nitrogen can be used regardless of the design features of the heating surfaces of the equipment.
To prevent standing corrosion of metal during major and current repairs, only conservation methods are applicable that make it possible to create a protective film on the metal surface that retains its properties for at least 1-2 months after draining the preservative solution, since emptying and depressurization of the system is inevitable. The validity period of the protective film on the metal surface after treating it with sodium nitrite can reach 3 months.
Preservation methods using water and reagent solutions are practically unacceptable for protecting boiler intermediate superheaters from standstill corrosion due to the difficulties associated with filling them and subsequent cleaning.
Methods for preserving hot water and steam boilers low pressure, as well as other equipment of closed technological circuits of heat and water supply, differ in many respects from the currently used methods for preventing stop-time corrosion at thermal power plants. Below we describe the main ways to prevent corrosion in the idle mode of equipment of devices of such circulation systems, taking into account the specifics of their operation.
Simplified preservation methods
It is advisable to use these methods for small boilers. They consist of completely removing water from the boilers and placing desiccant in them: calcined calcium chloride, quicklime, silica gel at the rate of 1-2 kg per 1 m 3 of volume.
This preservation method is suitable at room temperatures below and above zero. In rooms heated in winter time, one of the contact preservation methods can be implemented. It comes down to filling the entire internal volume of the unit with an alkaline solution (NaOH, Na 3 P0 4, etc.), ensuring complete stability of the protective film on the metal surface even when the liquid is saturated with oxygen.
Typically, solutions containing from 1.5-2 to 10 kg/m 3 NaOH or 5-20 kg/m 3 Na 3 P0 4 are used, depending on the content of neutral salts in the source water. Lower values apply to condensate, higher values apply to water containing up to 3000 mg/l of neutral salts.
Corrosion can also be prevented by the overpressure method, in which the steam pressure in the stopped unit is constantly maintained at a level above atmospheric pressure, and the water temperature remains above 100°C, which prevents the access of the main corrosive agent - oxygen.
An important condition for the effectiveness and efficiency of any method of protection is the maximum possible tightness of the steam-water fittings in order to avoid too rapid a decrease in pressure, loss of protective solution (or gas) or moisture ingress. In addition, in many cases, preliminary cleaning of surfaces from various deposits (salts, sludge, scale) is useful.
When implementing in various ways To protect against parking corrosion, the following must be kept in mind.
1. For all types of preservation, it is necessary to first remove (rinse) deposits of easily soluble salts (see above) in order to avoid increased parking corrosion in certain areas of the protected unit. It is mandatory to carry out this measure during contact conservation, otherwise intense local corrosion is possible.
2. For similar reasons, it is desirable to remove all types of insoluble deposits (sludge, scale, iron oxides) before long-term preservation.
3. If the valves are unreliable, it is necessary to disconnect the backup equipment from the operating units using plugs.
Leakage of steam and water is less dangerous with contact conservation, but is unacceptable with dry and gas protection methods.
The choice of desiccant is determined by the relative availability of the reagent and the desirability of obtaining the highest possible specific moisture capacity. The best desiccant is granular calcium chloride. Quicklime is much worse than calcium chloride, not only due to its lower moisture capacity, but also due to the rapid loss of its activity. Lime absorbs not only moisture from the air, but also carbon dioxide, as a result of which it becomes covered with a layer of calcium carbonate, which prevents further absorption of moisture.
Introduction
Corrosion (from Latin corrosio - corrosion) is the spontaneous destruction of metals as a result of chemical or physico-chemical interaction with the environment. In general, this is the destruction of any material - be it metal or ceramics, wood or polymer. The cause of corrosion is thermodynamic instability construction materials to the effects of substances in the environment in contact with them. Example - oxygen corrosion of iron in water:
4Fe + 2H 2 O + ZO 2 = 2 (Fe 2 O 3 H 2 O)
IN Everyday life For iron alloys (steels), the term “rusting” is more often used. Cases of corrosion of polymers are less known. In relation to them, there is the concept of “aging”, similar to the term “corrosion” for metals. For example, the aging of rubber due to interaction with atmospheric oxygen or the destruction of some plastics under the influence of precipitation, as well as biological corrosion. The rate of corrosion, like any chemical reaction, is very dependent on temperature. An increase in temperature of 100 degrees can increase the corrosion rate by several orders of magnitude.
Corrosion processes are characterized by a wide distribution and variety of conditions and environments in which it occurs. Therefore, there is no single and comprehensive classification of corrosion cases encountered. The main classification is made according to the mechanism of the process. There are two types: chemical corrosion and electrochemical corrosion. IN this essay Chemical corrosion is discussed in detail using the example of small and large-capacity ship boiler plants.
Corrosion processes are characterized by a wide distribution and variety of conditions and environments in which it occurs. Therefore, there is no single and comprehensive classification of corrosion cases encountered.
Type aggressive environments, in which the destruction process occurs, corrosion can be of the following types:
1) -Gas corrosion
2) - Corrosion in non-electrolytes
3) -Atmospheric corrosion
4) -Corrosion in electrolytes
5) -Underground corrosion
6) -Biocorrosion
7) - Corrosion by stray current.
According to the conditions of the corrosion process, the following types are distinguished:
1) - Contact corrosion
2) - Crevice corrosion
3) -Corrosion during partial immersion
4) -Corrosion during full immersion
5) -Corrosion during alternating immersion
6) -Friction corrosion
7) -Stress corrosion.
By nature of destruction:
Complete corrosion covering the entire surface:
1) - uniform;
2) - uneven;
3) -selective.
Local (local) corrosion covering individual areas:
1) - spots;
2) - ulcerative;
3) - spot (or pitting);
4) - through;
5) - intercrystalline.
Let's imagine metal in the process of producing rolled metal at a metallurgical plant: a red-hot mass moves along the stands of a rolling mill. Fiery splashes fly out from her in all directions. This is when particles of scale break off from the surface of the metal - a product of chemical corrosion resulting from the interaction of the metal with atmospheric oxygen. This process of spontaneous destruction of a metal due to the direct interaction of oxidizer particles and the oxidized metal is called chemical corrosion.
Chemical corrosion is the interaction of a metal surface with a (corrosive) environment, not accompanied by the occurrence of electrochemical processes at the phase boundary. In this case, the interactions of metal oxidation and reduction of the oxidizing component of the corrosive environment occur in one act. For example, the formation of scale when iron-based materials react at high temperatures with oxygen:
4Fe + 3O 2 → 2Fe 2 O 3
During electrochemical corrosion, the ionization of metal atoms and the reduction of the oxidizing component of the corrosive environment do not occur in one act and their rates depend on the electrode potential of the metal (for example, rusting of steel in sea water).
In chemical corrosion, metal oxidation and reduction of the oxidizing component of the corrosive environment occur simultaneously. Such corrosion is observed when metals are exposed to dry gases (air, fuel combustion products) and liquid non-electrolytes (oil, gasoline, etc.) and is a heterogeneous chemical reaction.
The process of chemical corrosion occurs as follows. The oxidizing component of the external environment, taking away valence electrons from the metal, simultaneously enters into a chemical compound with it, forming a film (corrosion product) on the metal surface. Further formation of the film occurs due to mutual two-way diffusion through the film of the aggressive medium towards the metal and metal atoms towards external environment and their interactions. Moreover, if the resulting film has protective properties, i.e., it prevents the diffusion of atoms, then corrosion proceeds with self-braking over time. Such a film is formed on copper at a heating temperature of 100 °C, on nickel at 650, on iron at 400 °C. Heating steel products above 600 °C leads to the formation of a loose film on their surface. With increasing temperature, the oxidation process accelerates.
The most common type of chemical corrosion is the corrosion of metals in gases at high temperatures - gas corrosion. Examples of such corrosion are oxidation of furnace fittings and engine parts internal combustion, grate bars, parts of kerosene lamps and oxidation during high-temperature processing of metals (forging, rolling, stamping). Other corrosion products may also form on the surface of metal products. For example, when exposed to sulfur compounds, sulfur compounds are formed on iron; on silver, when exposed to iodine vapor, silver iodide is formed, etc. However, most often a layer of oxide compounds is formed on the surface of metals.
Temperature has a great influence on the rate of chemical corrosion. As temperature increases, the rate of gas corrosion increases. The composition of the gas environment has a specific effect on the corrosion rate various metals. Thus, nickel is stable in an environment of oxygen and carbon dioxide, but is highly corroded in an atmosphere of sulfur dioxide. Copper is susceptible to corrosion in an oxygen atmosphere, but is stable in a sulfur dioxide atmosphere. Chromium is corrosion resistant in all three gas environments.
To protect against gas corrosion, heat-resistant alloying with chromium, aluminum and silicon is used, creating protective atmospheres and protective coatings aluminum, chromium, silicon and heat-resistant enamels.
2. Chemical corrosion in ship steam boilers.
Types of corrosion. During operation, the elements of a steam boiler are exposed to aggressive media - water, steam and flue gases. There are chemical and electrochemical corrosion.
Parts and components of machines operating at high temperatures are susceptible to chemical corrosion - piston and turbine engines, rocket engines etc. The chemical affinity of most metals for oxygen at high temperatures is almost unlimited, since the oxides of all technically important metals are able to dissolve in metals and leave the equilibrium system:
2Me(t) + O 2 (g) 2MeO(t); MeO(t) [MeO] (solution)Under these conditions, oxidation is always possible, but along with the dissolution of the oxide, an oxide layer also appears on the surface of the metal, which can inhibit the oxidation process.
The rate of metal oxidation depends on the rate of the chemical reaction itself and the rate of diffusion of the oxidizing agent through the film, and therefore protective effect The better the continuity of the film and the lower the diffusion capacity, the higher it is. The continuity of the film formed on the surface of the metal can be assessed by the ratio of the volume of the formed oxide or some other compound to the volume of the metal spent on the formation of this oxide (Pilling-Badwords factor). Coefficient a (Pilling-Badwords factor) y different metals It has different meanings. Metals that have a<1, не могут создавать сплошные оксидные слои, и через несплошности в слое (трещины) кислород свободно проникает к поверхности металла.
Continuous and stable oxide layers are formed at a = 1.2-1.6, but at large values of a the films are not continuous, easily separated from the metal surface (iron scale) as a result of internal stresses.
The Pilling-Badwords factor gives a very approximate estimate, since the composition of the oxide layers has a wide range of homogeneity, which is also reflected in the density of the oxide. So, for example, for chromium a = 2.02 (for pure phases), but the oxide film formed on it is very resistant to environmental influences. The thickness of the oxide film on the metal surface varies depending on time.
Chemical corrosion, caused by steam or water, destroys the metal evenly over the entire surface. The rate of such corrosion in modern marine boilers is low. More dangerous is local chemical corrosion caused by aggressive chemical compounds contained in ash deposits (sulfur, vanadium oxides, etc.).
Electrochemical corrosion, as its name indicates, is associated not only with chemical processes, but also with the movement of electrons in interacting media, i.e. with the appearance of electric current. These processes occur when the metal interacts with electrolyte solutions, which takes place in a steam boiler in which boiler water circulates, which is a solution of salts and alkalis that have disintegrated into ions. Electrochemical corrosion also occurs when the metal comes into contact with air (at normal temperature), which always contains water vapor, which condenses on the surface of the metal in the form of a thin film of moisture, creating conditions for electrochemical corrosion to occur.
Owners of patent RU 2503747:
TECHNICAL FIELD
The invention relates to heat power engineering and can be used to protect heating pipes of steam and hot water boilers, heat exchangers, boiler units, evaporators, heating mains, heating systems of residential buildings and industrial facilities from scale during ongoing operation.
BACKGROUND OF THE ART
The operation of steam boilers is associated with simultaneous exposure to high temperatures, pressure, mechanical stress and an aggressive environment, which is boiler water. Boiler water and the metal of the boiler heating surfaces are separate phases of a complex system that is formed upon their contact. The result of the interaction of these phases is surface processes that occur at their interface. As a result of this, corrosion and scale formation occur in the metal of the heating surfaces, which leads to a change in the structure and mechanical properties of the metal, and which contributes to the development of various damages. Since the thermal conductivity of scale is fifty times lower than that of iron heating pipes, there are losses of thermal energy during heat transfer - with a scale thickness of 1 mm from 7 to 12%, and with 3 mm - 25%. Severe scale formation in a continuous steam boiler system often causes production to be shut down for several days each year to remove the scale.
The quality of feed water and, therefore, boiler water is determined by the presence of impurities that can cause various types of corrosion of the metal of internal heating surfaces, the formation of primary scale on them, as well as sludge as a source of secondary scale formation. In addition, the quality of boiler water also depends on the properties of substances formed as a result of surface phenomena during water transportation and condensate through pipelines during water treatment processes. Removing impurities from feed water is one of the ways to prevent the formation of scale and corrosion and is carried out by methods of preliminary (pre-boiler) water treatment, which are aimed at maximizing the removal of impurities found in the source water. However, the methods used do not allow us to completely eliminate the content of impurities in water, which is associated not only with technical difficulties, but also with the economic feasibility of using pre-boiler water treatment methods. In addition, since water treatment is a complex technical system, it is redundant for boilers of low and medium capacity.
Known methods for removing already formed deposits mainly use mechanical and chemical cleaning methods. The disadvantage of these methods is that they cannot be produced during the operation of the boilers. In addition, chemical cleaning methods often require the use of expensive chemicals.
There are also known methods to prevent the formation of scale and corrosion, carried out during the operation of boilers.
US Pat. No. 1,877,389 proposes a method for removing scale and preventing its formation in hot water and steam boilers. In this method, the surface of the boiler is the cathode, and the anode is placed inside the pipeline. The method involves passing direct or alternating current through the system. The authors note that the mechanism of action of the method is that under the influence of an electric current, gas bubbles form on the surface of the boiler, which lead to the peeling off of existing scale and prevent the formation of a new one. The disadvantage of this method is the need to constantly maintain the flow of electric current in the system.
US Pat. No. 5,667,677 proposes a method for treating a liquid, particularly water, in a pipeline to slow down the formation of scale. This method is based on the creation of an electromagnetic field in pipes, which repels calcium and magnesium ions dissolved in water from the walls of pipes and equipment, preventing them from crystallizing in the form of scale, which allows the operation of boilers, boilers, heat exchangers, and cooling systems on hard water. The disadvantage of this method is the high cost and complexity of the equipment used.
Application WO 2004016833 proposes a method for reducing the formation of scale on a metal surface exposed to a supersaturated alkaline aqueous solution which is capable of forming scale after a period of exposure, comprising applying a cathodic potential to said surface.
This method can be used in various technological processes in which the metal is in contact with an aqueous solution, in particular in heat exchangers. The disadvantage of this method is that it does not protect the metal surface from corrosion after removing the cathodic potential.
Thus, there is currently a need to develop an improved method for preventing scale formation of heating pipes, hot water boilers and steam boilers, which would be economical and highly effective and provide anti-corrosion protection to the surface for a long period of time after exposure.
In the present invention, this problem is solved using a method according to which a current-carrying electric potential is created on a metal surface, sufficient to neutralize the electrostatic component of the adhesion force of colloidal particles and ions to the metal surface.
BRIEF DESCRIPTION OF THE INVENTION
An object of the present invention is to provide an improved method for preventing the formation of scale in heating pipes of hot water and steam boilers.
Another objective of the present invention is to provide the possibility of eliminating or significantly reducing the need for descaling during operation of hot water and steam boilers.
Another objective of the present invention is to eliminate the need to use consumable reagents to prevent the formation of scale and corrosion of heating pipes of water heating and steam boilers.
Another object of the present invention is to enable work to begin to prevent the formation of scale and corrosion of heating pipes of hot water and steam boilers on contaminated boiler pipes.
The present invention relates to a method for preventing the formation of scale and corrosion on a metal surface made of an iron-containing alloy and in contact with a steam-water environment from which scale is capable of forming. This method consists in applying to the specified metal surface a current-carrying electric potential sufficient to neutralize the electrostatic component of the adhesion force of colloidal particles and ions to the metal surface.
According to some private embodiments of the claimed method, the current-carrying potential is set in the range of 61-150 V. According to some private embodiments of the claimed method, the above iron-containing alloy is steel. In some embodiments, the metal surface is the interior surface of the heating tubes of a hot water or steam boiler.
The method disclosed herein has the following advantages. One advantage of the method is reduced scale formation. Another advantage of the present invention is the ability to use a working electrophysical apparatus once purchased without the need to use consumable synthetic reagents. Another advantage is the possibility of starting work on dirty boiler tubes.
The technical result of the present invention, therefore, is to increase the operating efficiency of hot water and steam boilers, increase productivity, increase heat transfer efficiency, reduce fuel consumption for heating the boiler, save energy, etc.
Other technical results and advantages of the present invention include providing the possibility of layer-by-layer destruction and removal of already formed scale, as well as preventing its new formation.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 shows the distribution of deposits on the internal surfaces of the boiler as a result of applying the method according to the present invention.
DETAILED DESCRIPTION OF THE INVENTION
The method of the present invention involves applying to a metal surface subject to scale formation a current-carrying electrical potential sufficient to neutralize the electrostatic component of the adhesion force of colloidal particles and scale-forming ions to the metal surface.
The term "conducting electrical potential" as used in this application means an alternating potential that neutralizes the electrical double layer at the interface of the metal and the steam-water medium containing salts that lead to scale formation.
As is known to a person skilled in the art, the carriers of electric charge in a metal, slow compared to the main charge carriers - electrons, are dislocations of its crystal structure, which carry an electric charge and form dislocation currents. Coming to the surface of the heating pipes of the boiler, these currents become part of the double electrical layer during the formation of scale. The current-carrying, electrical, pulsating (i.e., alternating) potential initiates the removal of the electrical charge of dislocations from the metal surface to the ground. In this respect, it is a conductor of dislocation currents. As a result of the action of this current-carrying electrical potential, the double electrical layer is destroyed, and the scale gradually disintegrates and passes into the boiler water in the form of sludge, which is removed from the boiler during periodic purging.
Thus, the term “current-carrying potential” is understandable to a person skilled in the art and, in addition, is known from the prior art (see, for example, patent RU 2128804 C1).
As a device for creating a current-carrying electrical potential, for example, a device described in RU 2100492 C1 can be used, which includes a converter with a frequency converter and a pulsating potential regulator, as well as a pulse shape regulator. A detailed description of this device is given in RU 2100492 C1. Any other similar device may also be used, as will be appreciated by one skilled in the art.
The conductive electrical potential according to the present invention can be applied to any part of the metal surface remote from the base of the boiler. The place of application is determined by the convenience and/or effectiveness of using the claimed method. One skilled in the art, using the information disclosed herein and using standard testing techniques, will be able to determine the optimal location for application of the current-sinking electrical potential.
In some embodiments of the present invention, the current-sinking electrical potential is variable.
The current-sinking electric potential according to the present invention can be applied for various periods of time. The time of application of the potential is determined by the nature and degree of contamination of the metal surface, the composition of the water used, the temperature regime and the operating characteristics of the heating device and other factors known to specialists in this field of technology. One skilled in the art, using the information disclosed herein and using standard test procedures, will be able to determine the optimal time to apply the current-sinking electrical potential based on the objectives, conditions, and condition of the thermal device.
The magnitude of the current-carrying potential required to neutralize the electrostatic component of the adhesion force can be determined by a specialist in the field of colloid chemistry based on information known from the prior art, for example from the book B.V. Deryagin, N.V. Churaev, V.M. Muller. "Surface Forces", Moscow, "Nauka", 1985. According to some embodiments, the magnitude of the current-carrying electrical potential is in the range from 10 V to 200 V, more preferably from 60 V to 150 V, even more preferably from 61 V to 150 V. Values of the current-carrying electrical potential in the range from 61 V to 150 V lead to the discharge of the double electrical layer, which is the basis of the electrostatic component of the adhesion forces in scale and, as a consequence, destruction of scale. Values of the current-carrying potential below 61 V are insufficient to destroy scale, and at values of the current-carrying potential above 150 V, unwanted electrical erosion destruction of the metal of the heating tubes is likely to begin.
The metal surface to which the method according to the present invention can be applied can be part of the following thermal devices: heating pipes of steam and hot water boilers, heat exchangers, boiler units, evaporators, heating mains, heating systems of residential buildings and industrial facilities during ongoing operation. This list is illustrative and does not limit the list of devices to which the method according to the present invention can be applied.
In some embodiments, the iron-containing alloy from which the metal surface is made to which the method of the present invention can be applied may be steel or other iron-containing material such as cast iron, kovar, fechral, transformer steel, alsifer, magneto, alnico, chromium steel, invar, etc. This list is illustrative and does not limit the list of iron-containing alloys to which the method according to the present invention can be applied. One skilled in the art, based on knowledge known in the art, will be able to identify such iron-containing alloys that can be used according to the present invention.
The aqueous medium from which scale is capable of forming, according to some embodiments of the present invention, is tap water. The aqueous medium may also be water containing dissolved metal compounds. The dissolved metal compounds may be iron and/or alkaline earth metal compounds. The aqueous medium may also be an aqueous suspension of colloidal particles of iron and/or alkaline earth metal compounds.
The method according to the present invention removes previously formed deposits and serves as a reagent-free means of cleaning internal surfaces during operation of a heating device, subsequently ensuring its scale-free operation. In this case, the size of the zone within which the prevention of scale and corrosion is achieved significantly exceeds the size of the zone of effective scale destruction.
The method according to the present invention has the following advantages:
Does not require the use of reagents, i.e. environmentally friendly;
Easy to implement, does not require special devices;
Allows you to increase the heat transfer coefficient and increase the efficiency of boilers, which significantly affects the economic indicators of its operation;
Can be used as an addition to the applied methods of pre-boiler water treatment, or separately;
Allows you to abandon the processes of water softening and deaeration, which greatly simplifies the technological scheme of boiler houses and makes it possible to significantly reduce costs during construction and operation.
Possible objects of the method may be hot water boilers, waste heat boilers, closed heat supply systems, installations for thermal desalination of sea water, steam conversion plants, etc.
The absence of corrosion damage and scale formation on internal surfaces opens up the possibility of developing fundamentally new design and layout solutions for low- and medium-power steam boilers. This will allow, due to the intensification of thermal processes, to achieve a significant reduction in the weight and dimensions of steam boilers. Ensure the specified temperature level of heating surfaces and, consequently, reduce fuel consumption, the volume of flue gases and reduce their emissions into the atmosphere.
EXAMPLE OF IMPLEMENTATION
The method claimed in the present invention was tested at the Admiralty Shipyards and Krasny Khimik boiler plants. The method according to the present invention has been shown to effectively clean the internal surfaces of boiler units from deposits. In the course of these works, fuel equivalent savings of 3-10% were obtained, while the variation in savings values is associated with varying degrees of contamination of the internal surfaces of the boiler units. The purpose of the work was to evaluate the effectiveness of the claimed method for ensuring reagent-free, scale-free operation of medium-power steam boilers under conditions of high-quality water treatment, compliance with the water chemistry regime and a high professional level of equipment operation.
The method claimed in the present invention was tested on steam boiler unit No. 3 DKVR 20/13 of the 4th Krasnoselskaya boiler house of the South-Western branch of the State Unitary Enterprise "TEK SPb". The operation of the boiler unit was carried out in strict accordance with the requirements of regulatory documents. The boiler is equipped with all the necessary means of monitoring its operating parameters (pressure and flow rate of generated steam, temperature and flow rate of feed water, pressure of blast air and fuel on the burners, vacuum in the main sections of the gas path of the boiler unit). The steam output of the boiler was maintained at 18 t/hour, the steam pressure in the boiler drum was 8.1...8.3 kg/cm 2 . The economizer operated in heating mode. City water supply water was used as the source water, which met the requirements of GOST 2874-82 “Drinking water”. It should be noted that the amount of iron compounds entering the specified boiler room, as a rule, exceeds regulatory requirements (0.3 mg/l) and amounts to 0.3-0.5 mg/l, which leads to intensive overgrowing of internal surfaces with ferrous compounds.
The effectiveness of the method was assessed based on the condition of the internal surfaces of the boiler unit.
Assessment of the influence of the method according to the present invention on the condition of the internal heating surfaces of the boiler unit.
Before the start of the tests, an internal inspection of the boiler unit was carried out and the initial condition of the internal surfaces was recorded. A preliminary inspection of the boiler was carried out at the beginning of the heating season, a month after its chemical cleaning. As a result of the inspection, it was revealed: on the surface of the drums there are continuous solid deposits of a dark brown color, possessing paramagnetic properties and presumably consisting of iron oxides. The thickness of the deposits was up to 0.4 mm visually. In the visible part of the boiling pipes, mainly on the side facing the furnace, non-continuous solid deposits were found (up to five spots per 100 mm of pipe length with a size of 2 to 15 mm and a visual thickness of up to 0.5 mm).
The device for creating a current-carrying potential, described in RU 2100492 C1, was connected at point (1) to the hatch (2) of the upper drum on the back side of the boiler (see Fig. 1). The current-carrying electric potential was equal to 100 V. The current-carrying electric potential was maintained continuously for 1.5 months. At the end of this period, the boiler unit was opened. As a result of an internal inspection of the boiler unit, an almost complete absence of deposits (no more than 0.1 mm visually) was established on the surface (3) of the upper and lower drums within 2-2.5 meters (zone (4)) from the drum hatches (device connection points to create a current-carrying potential (1)). At a distance of 2.5-3.0 m (zone (5)) from the hatches, deposits (6) were preserved in the form of individual tubercles (spots) up to 0.3 mm thick (see Fig. 1). Further, as you move towards the front, (at a distance of 3.0-3.5 m from the hatches) continuous deposits begin (7) up to 0.4 mm visually, i.e. at this distance from the connection point of the device, the effect of the cleaning method according to the present invention was practically not evident. The current-carrying electric potential was equal to 100 V. The current-carrying electric potential was maintained continuously for 1.5 months. At the end of this period, the boiler unit was opened. As a result of an internal inspection of the boiler unit, an almost complete absence of deposits (no more than 0.1 mm visually) was established on the surface of the upper and lower drums within 2-2.5 meters from the drum hatches (attachment points of the device for creating current-carrying potential). At a distance of 2.5-3.0 m from the hatches, the deposits were preserved in the form of individual tubercles (spots) up to 0.3 mm thick (see Fig. 1). Further, as you move towards the front (at a distance of 3.0-3.5 m from the hatches), continuous deposits of up to 0.4 mm visually begin, i.e. at this distance from the connection point of the device, the effect of the cleaning method according to the present invention was practically not evident.
In the visible part of the boiling pipes, within 3.5-4.0 m from the drum hatches, an almost complete absence of deposits was observed. Further, as we move towards the front, non-continuous solid deposits are found (up to five spots per 100 l.mm with a size from 2 to 15 mm and a visual thickness of up to 0.5 mm).
As a result of this stage of testing, it was concluded that the method according to the present invention, without the use of any reagents, can effectively destroy previously formed deposits and ensure scale-free operation of the boiler unit.
At the next stage of testing, the device for creating a current-carrying potential was connected at point “B” and the tests continued for another 30-45 days.
The next opening of the boiler unit was carried out after 3.5 months of continuous operation of the device.
An inspection of the boiler unit showed that the previously remaining deposits were completely destroyed and only a small amount remained in the lower sections of the boiler pipes.
This allowed us to draw the following conclusions:
The size of the zone within which scale-free operation of the boiler unit is ensured significantly exceeds the size of the zone of effective destruction of deposits, which allows subsequent transfer of the point of connection of the current-carrying potential to clean the entire internal surface of the boiler unit and further maintain its scale-free operation mode;
The destruction of previously formed deposits and the prevention of the formation of new ones is ensured by processes of different nature.
Based on the results of the inspection, it was decided to continue testing until the end of the heating period in order to finally clean the drums and boiling pipes and determine the reliability of ensuring scale-free operation of the boiler. The next opening of the boiler unit was carried out after 210 days.
The results of the internal inspection of the boiler showed that the process of cleaning the internal surfaces of the boiler within the upper and lower drums and boiling pipes resulted in almost complete removal of deposits. A thin, dense coating formed on the entire surface of the metal, black in color with a blue tarnish, the thickness of which, even in a moistened state (almost immediately after opening the boiler), did not visually exceed 0.1 mm.
At the same time, the reliability of ensuring scale-free operation of the boiler unit when using the method of the present invention was confirmed.
The protective effect of the magnetite film lasted up to 2 months after disconnecting the device, which is quite enough to ensure the preservation of the boiler unit using the dry method when transferring it to reserve or for repairs.
Although the present invention has been described with respect to various specific examples and embodiments, it is to be understood that the invention is not limited thereto and that it may be practiced within the scope of the following claims.
1. A method for preventing the formation of scale on a metal surface made of an iron-containing alloy and in contact with a steam-water environment from which scale can form, including applying a current-carrying electric potential to said metal surface in the range from 61 V to 150 V to neutralize the electrostatic component of the force adhesion between said metal surface and colloidal particles and ions forming scale.
The invention relates to heat power engineering and can be used to protect against scale and corrosion heating pipes of steam and hot water boilers, heat exchangers, boiler units, evaporators, heating mains, heating systems of residential buildings and industrial facilities during operation. A method for preventing the formation of scale on a metal surface made of an iron-containing alloy and in contact with a steam-water environment from which scale is capable of forming involves applying to said metal surface a current-carrying electric potential in the range from 61 V to 150 V to neutralize the electrostatic component of the adhesion force between the specified metal surface and colloidal particles and ions forming scale. The technical result is increasing the efficiency and productivity of hot water and steam boilers, increasing the efficiency of heat transfer, ensuring layer-by-layer destruction and removal of formed scale, as well as preventing its new formation. 2 salary f-ly, 1 ave., 1 ill.
Accidents of steam boilers associated with violation of the water regime, corrosion and erosion of metal
Normal water regime is one of the most important conditions for the reliability and efficiency of operation of a boiler installation. The use of water with increased hardness to feed boilers entails the formation of scale, excessive fuel consumption and increased costs for repairs and cleaning of boilers. It is known that scale formation can lead to a steam boiler failure due to burnout of heating surfaces. Therefore, the correct water regime in the boiler room should be considered not only from the point of view of increasing the efficiency of the boiler installation, but also as the most important preventive measure to combat accidents.
Currently, boiler plants of industrial enterprises are equipped with water treatment devices, so their operating conditions have improved and the number of accidents caused by scale formation and corrosion has significantly decreased.
However, at some enterprises, the administration, having formally fulfilled the requirement of the Boiler Inspection Rules to equip boilers with water treatment units, does not provide normal operating conditions for these installations, does not control the quality of feed water and the condition of the boiler heating surfaces, allowing the boilers to become contaminated with scale and sludge. Here are some examples of boiler failures for these reasons.
1. In the boiler room of a prefabricated reinforced concrete structure plant, due to violations of the water regime in the boiler DKVR-6, 5-13, three screen pipes ruptured, some of the screen pipes were deformed, and bulges formed on many pipes.
The boiler room has a two-stage sodium cation exchange water treatment and deaerator, but the normal operation of the water treatment equipment was not given due attention. Regeneration of cation exchange filters was not carried out within the time limits established by the instructions, the quality of feed and boiler water was rarely checked, and the time limits for periodic boiler purging were not observed. The water in the deaerator was not heated to the required temperature and therefore deoxygenation of the water did not actually occur.
It was also established that raw water was often supplied to the boiler, while the requirements of the “Rules for the Design and Safe Operation of Steam and Hot Water Boilers” were not observed, according to which the shut-off devices on the raw water line must be sealed in the closed position, and each case of raw water supply must be recorded in the water treatment log. From individual entries in the water treatment log it is clear that the hardness of the feed water reached 2 mEq/kg or more, while the permissible value according to boiler inspection standards is 0.02 mEq/kg. Most often, the following entries were made in the log: “the water is dirty, hard,” without indicating the results of a chemical analysis of the water.
When inspecting the boiler after shutdown, deposits up to 5 mm thick were found on the internal surfaces of the screen pipes; individual pipes were almost completely clogged with scale and sludge. On the inner surface of the drum in the lower part, the thickness of deposits reached 3 mm, the front part of the drum is filled with sludge to one third of its height.
In 11 months Before this accident, similar damage (“cracks, dents, deformation) was detected in 13 boiler screen pipes. The defective pipes were replaced, but the administration of the enterprise, in violation of the “Instructions for the investigation of accidents that resulted in accidents at enterprises and facilities controlled by the USSR State Technical Supervision Committee,” did not investigate this case and did not take measures to improve the operating conditions of the boilers.
2. On the power train, raw water to feed a single-drum water-tube shielded steam boiler with a capacity of 10 t/h and an operating pressure of 41 kgf/cm2 was treated by the cation exchange method. Due to unsatisfactory performance of the cation and waste filter, the residual hardness of the softened water reached
0.7 mEq/kg instead of the 0.01 mEq/kg envisaged by the project. The boiler was not blown regularly. When stopping for repairs, the boiler drum and screen collectors were not opened or inspected. Due to scale deposits, a pipe ruptured, and a fireman was burned by steam and burning fuel ejected from the firebox.
The accident could not have happened if the boiler combustion door had been closed with a latch, as required by the rules for the safe operation of boilers.
3. At the cement plant, a newly installed single-drum water-tube boiler with a capacity of 35 t/h and a working pressure of 43 kgf/cm2 was put into operation without chemical water treatment, the installation of which had not been completed by that time. For a month, the boiler was fed with untreated water. The water was not deaerated for more than two months, since the steam line was not connected to the deaerator.
Violations of the water regime were allowed even after... pre-production equipment was put into operation. The boiler was often fed with raw water; the purge regime was not followed; the chemical laboratory did not control the quality of the feed water, since it was not equipped with the necessary reagents.
Due to unsatisfactory water conditions, deposits on the internal surfaces of the screen pipes reached a thickness of 8 mm; As a result, bulges formed on 36 screen pipes; a significant part of the pipes was deformed, and the drum walls on the inside were corroded.
4. At the reinforced concrete products plant, the boiler of the Shukhov-Berlin system was powered by electromagnetically treated water. It is known that with this method of water treatment, timely and effective removal of sludge from the boiler must be ensured.
However, during operation of the boiler this condition was not met. The boiler was not purged regularly, and the boiler shutdown schedule for flushing and cleaning was not followed.
As a result, a large amount of sludge accumulated inside the boiler. The back part of the pipes was clogged with sludge at 70-80% of the cross-section, the mud trap - at 70% of the volume, the thickness of scale on the heating surfaces reached 4 mm. This led to overheating and deformation of the boiling pipes, pipe pipes and heads of tubular sections.
When choosing an electromagnetic method for processing iodine in this case, the quality of the feed water and the design features of the boiler were not taken into account, and no measures were taken to organize a normal blowing regime, which led to the accumulation of sludge and significant scale deposits in the boiler.
5. The issues of organizing a rational water regime to ensure reliable and economical operation of boilers at thermal power plants have acquired exceptional importance.
The formation of deposits on the heating surfaces of boiler units occurs as a result of complex physicochemical processes in which not only scale formers, but also metal oxides and easily soluble compounds are involved. Dialysis of deposits shows that, along with scale-forming salts, they contain a significant amount of iron oxides, which are products of corrosion processes.
Over the past years, our country has achieved significant progress in organizing the rational water regime of thermal power plant boilers and chemical control of water and steam, as well as in the introduction of corrosion-resistant metals and protective coatings.
The use of modern water treatment means has dramatically increased the reliability and cost-effectiveness of operating power equipment.
However, at some thermal power plants, violations of the water regime are still allowed.
In June 1976, for this reason, at the thermal power plant of the pulp and paper mill, an accident occurred on a steam boiler type BKZ-220-100 f with a steam capacity of 220 t/h with steam parameters of 100 kgf/cm2 and 540 ° C, manufactured at the Barnaul Boiler Plant in 1964 d. Single-drum boiler with natural circulation, made according to a U-shaped design. The prismatic combustion chamber is completely shielded by pipes with an outer diameter of 60 mm, the pitch of which is 64 mm. The lower part of the screen surface forms a so-called cold funnel, along the slopes of which particles of slag in solid form roll down into the slag chest. The evaporation scheme is two-stage, with steam flushed with feed water. The first evaporation stage is included directly in the boiler drum, the second stage is remote steam separation cyclones included in the circulation circuit of the middle side screen blocks.
The boiler is fed with a mixture of chemically purified water (60%) and condensate coming from turbines and production shops (40%). Water for feeding the boiler is processed according to the following scheme: limestone - coagulation - magnesium desiliconization in
Clarifiers - two-stage cationization.
The boiler runs on coal from the Inta deposit with a relatively low ash melting point. Fuel oil is used as starting fuel. Before the accident, the boiler operated for 73,300 hours.
On the day of the accident, the boiler was turned on at 00:45 and operated without deviation from normal mode until 14:00. The pressure in the drum during this period of operation was maintained within the range of 84-102 kgf/cm2, steam consumption was 145-180 t/h, temperature superheated steam -520-535° C.
At 14:10, 11 pipes of the front screen ruptured in the cold funnel area at 3.7 m with partial destruction
lining. It is believed that a water pipe or two ruptured first, followed by the rupture of other pipes. The water level dropped sharply and the boiler was stopped by automatic protection.
The inspection showed that the inclined sections of the pipes of the cold funnel outside the bends were destroyed, while two pipes were torn off from the first front lower collector, and nine from the second. The rupture is brittle; the edges at the rupture sites are blunt and not thinned. The length of the ruptured sections of pipes ranges from one to three meters. On the inner surface of damaged pipes, as well as samples cut from undamaged pipes, loose deposits up to 2.5 mm thick were found, as well as a large number of pits, up to 2 mm deep, located in a chain up to 10 mm wide along two generatrices along the heating boundary of the pipe. It was in places of corrosion damage that the metal was destroyed.
During the investigation of the accident, it turned out that earlier during the operation of the boiler there had already been ruptures of the screen pipes. For example, two months before the accident, a front screen pipe ruptured at 6.0 m. After 3 days, the boiler was shut down again due to the rupture of two front screen pipes at 7.0 m. And in these cases, the destruction of the pipes was result of corrosion damage to the metal.
In accordance with the approved schedule, the boiler was to be shut down for major repairs in the third quarter of 1976. During the repair period, it was planned to replace the front screen pipes in the area of the cold funnel. However, the boiler was not stopped for repairs and the pipes were not replaced.
Corrosion damage to the metal was a consequence of violations of the water regime, which were allowed for a long time during the operation of the boilers of the thermal power plant. The boilers were fed with water with a high content of iron, copper and oxygen. The total salt content in the feed water significantly exceeded the permissible standards, as a result of which, even in the circuits of the first evaporation stage, the salt content reached 800 mg/kg. Industrial condensates with an iron content of 400-600 mg/kg used to feed boilers were not purified. For this reason, and also because there was not sufficient anti-corrosion protection of the water treatment equipment (protection was partially carried out), there were significant deposits on the internal surfaces of the pipes (up to 1000 g/m2), mainly consisting of iron compounds. Amination and hydrazination of feed water was introduced only shortly before the accident. Pre-startup and operational acid flushing of the boilers was not carried out.
Other violations of the Rules for the Technical Operation of Boilers also contributed to the accident. At thermal power plants, boilers are lit very often, and the largest number of kindlings occurred in the boiler with which the accident occurred. The boilers are equipped with devices for steam heating, but they were not used for kindling. During kindling, the movements of the screen collectors were not controlled.
To clarify the nature of the corrosion process and determine the reasons for the formation of pits mainly in the first two panels of the front screen and the location of these pits in the form of chains, the materials of the accident investigation were sent to the CKTI. When reviewing these materials, attention was drawn to the fact that
the boilers operated with sharply variable loads, and a significant reduction in steam output was allowed (up to 90 t/h), which could lead to local circulation disruption. The boilers were heated in the following way: at the beginning of the kindling, two nozzles located opposite (diagonally) were turned on. This method led to a slowdown in the process of natural circulation in the panels of the first and second front screens. It is in these screens that the main focus of ulcerative lesions is found. Nitrites occasionally appeared in the feed water, the concentration of which was not monitored.
An analysis of the accident materials, taking into account the listed shortcomings, gave reason to believe that the formation of chains of ulcers on the side generatrices of the internal surfaces of the front screen pipes on the slope of the cold funnel is the result of a long-term process of sub-sludge electrochemical corrosion. The depolarizers of this process were nitrites and oxygen dissolved in water.
The arrangement of pits in the form of chains is, apparently, the result of the boiler operating during kindling with an unsteady process of natural circulation. During the period of the beginning of circulation, pore bubbles periodically form on the upper generatrix of the inclined pipes of the cold funnel, causing the effect of local thermal pulsations in the metal through the occurrence of electrochemical processes in the region of temporary phase separation. It was these places that became the foci for the formation of chains of ulcers. The predominant formation of pitting in the first two panels of the front screen was a consequence of improper kindling conditions.
6. At the TIC WB, during the operation of the PK-YUSH-2 boiler with a steam production capacity of 230 t/h with steam parameters of 100 kgf/cm2 and 540° C, steaming was noticed at the outlet from the fresh steam collection manifold to the main safety valve. The outlet is connected by welding to a cast tee welded into the prefabricated manifold.
The boiler was emergency stopped. During the inspection, an annular crack was discovered in the lower part of the pipe (168X13 mm) of the horizontal section of the bend in the immediate vicinity of the place where the bend is connected to the cast tee. The length of the crack on the outer surface is 70 mm and on the inner surface is 110 mm. On the inner surface of the pipe at the site of its damage, a large number of corrosion pits and individual cracks located parallel to the main one were revealed.
Metallographic analysis established that the cracks begin from pits in the decarbonized metal layer and then develop transcrystalline in the direction perpendicular to the surface of the pipe. The microstructure of the pipe metal is ferrite grains and thin pearlite chains along the grain boundaries. According to the scale given as an appendix to MRTU 14-4-21-67, the microstructure can be assessed with a score of 8.
The chemical composition of the metal of the damaged pipe corresponds to steel 12Х1МФ. Mechanical properties meet the requirements of the technical delivery conditions. The diameter of the pipe in the damaged area does not exceed the plus tolerance.
The horizontal outlet to the safety valve with an unregulated fastening system can be considered as a cantilever beam welded to a tee rigidly fixed in the manifold, with maximum bending stresses at the sealing point, i.e. in the area where the pipe has been damaged. With absence
drainage in the outlet and the presence of a counter slope, due to elastic bending in the area from the safety valve to the fresh steam collection manifold, in the lower part of the pipe in front of the tee there may be a constant accumulation of a small amount of condensate, enriched with oxygen during shutdowns, conservation and commissioning of the boiler from the air. Under these conditions, corrosive erosion of the metal occurred, and the combined effect of condensate and tensile stresses on the metal caused its corrosion cracking. During operation, fatigue-corrosion cracks can develop in places of corrosion pits and shallow cracks as a result of aggressive environmental influences and alternating stresses in the metal, which apparently happened in this case.
To prevent condensate from accumulating, reverse steam circulation was installed in the outlet. To do this, the outlet pipe directly in front of the main safety valve was connected by a heating line (pipes with a diameter of 10 mm) to the intermediate chamber of the superheater, through which steam is supplied at a temperature of 430 ° C. With a small difference in excess pressure (up to 4 kgf/cm2), continuous steam flow is ensured and the temperature of the medium in the outlet is maintained at least 400° C. Reconstruction of the outlet was carried out on all boilers of PK-YUSH-2 CHPP.
In order to prevent damage to the outlets to the main safety valves on PK-YUSH-2 boilers and similar ones, it is recommended:
Ultrasound check the lower semi-perimeters of the branch pipes at the points of welding to the tees;
Check whether the required slopes are observed and, if necessary, adjust the systems for attaching steam pipelines to the main safety valves, taking into account the actual condition of the steam pipelines (insulation weight, actual weight of pipes, previously carried out reconstructions);
Make reverse steam circulation in the outlets to the main safety valves; the design and internal diameter of the heating steam pipeline in each individual case must be agreed with the equipment manufacturer;
All dead-end branches to safety valves must be carefully insulated.
(From express information from STSNTI ORGRES - 1975)