A number of power plants use river and tap water with low pH and low hardness. Additional processing river water at a waterworks usually leads to a decrease in pH, a decrease in alkalinity and an increase in the content of aggressive carbon dioxide. The appearance of aggressive carbon dioxide is also possible in acidification schemes used for large heat supply systems with direct water supply hot water(2000–3000 t/h). Softening water according to the Na-cationization scheme increases its aggressiveness due to the removal of natural corrosion inhibitors - hardness salts.
With poorly established water deaeration and possible increases in oxygen and carbon dioxide concentrations due to the lack of additional protective measures in heat supply systems, pipelines, heat exchangers, battery tanks and other equipment.
It is known that an increase in temperature promotes the development of corrosion processes that occur both with the absorption of oxygen and with the release of hydrogen. With an increase in temperature above 40 °C, oxygen and carbon dioxide forms of corrosion increase sharply.
Special view subsludge corrosion occurs under conditions of low residual oxygen content (if PTE standards are met) and when the amount of iron oxides is more than 400 μg/dm 3 (in terms of Fe). This type of corrosion, previously known in the practice of operating steam boilers, was discovered under conditions of relatively weak heating and the absence of thermal loads. In this case, loose corrosion products, consisting mainly of hydrated ferric oxides, are active depolarizers of the cathodic process.
When operating heating equipment, crevice corrosion is often observed, i.e., selective, intense corrosion destruction of metal in a crevice (gap). A feature of the processes occurring in narrow gaps is a reduced oxygen concentration compared to the concentration in the solution volume and a slow removal of corrosion reaction products. As a result of the accumulation of the latter and their hydrolysis, a decrease in the pH of the solution in the gap is possible.
With constant replenishment of a heating network with an open water supply with deaerated water, the possibility of the formation of through fistulas on pipelines is completely eliminated only under normal hydraulic conditions, when at all points of the heating supply system the overpressure above atmospheric.
The causes of pitting corrosion of hot water boiler pipes and other equipment are as follows: poor deaeration of make-up water; low pH value due to the presence of aggressive carbon dioxide (up to 10–15 mg/dm 3); accumulation of oxygen corrosion products of iron (Fe 2 O 3) on heat transfer surfaces. An increased content of iron oxides in network water contributes to the contamination of boiler heating surfaces with iron oxide deposits.
A number of researchers recognize the important role in the occurrence of subsludge corrosion of the process of rusting pipes of hot water boilers during their downtime, when proper measures have not been taken to prevent standstill corrosion. Foci of corrosion arising from exposure to wet surfaces of boilers atmospheric air, continue to function when the boilers are operating.
A number of boiler houses use river and tap water with a low pH value and low hardness to feed heating networks. Additional treatment of river water at a waterworks usually leads to a decrease in pH, a decrease in alkalinity and an increase in the content of aggressive carbon dioxide. The appearance of aggressive carbon dioxide is also possible in connection schemes used for large heat supply systems with direct hot water supply (2000-3000 t/h). Softening water according to the Na-cationization scheme increases its aggressiveness due to the removal of natural corrosion inhibitors - hardness salts.
With poorly established water deaeration and possible increases in oxygen and carbon dioxide concentrations, due to the lack of additional protective measures in heat supply systems, thermal power equipment of thermal power plants is susceptible to internal corrosion.
When examining the make-up tract of one of the thermal power plants in Leningrad, the following data were obtained on the corrosion rate, g/(m2 4):
Installation location of corrosion indicators
In the make-up water pipeline after the heaters of the heating network in front of the deaerators, the 7 mm thick pipes thinned over the year of operation, in some places up to 1 mm, and through fistulas formed in some areas.
The causes of pitting corrosion of hot water boiler pipes are as follows:
insufficient removal of oxygen from make-up water;
low pH value due to the presence of aggressive carbon dioxide
(up to 10h15 mg/l);
accumulation of oxygen corrosion products of iron (Fe2O3;) on heat transfer surfaces.
Operating equipment on network water with an iron concentration of over 600 µg/l usually results in intensive (over 1000 g/m2) contamination of their heating surfaces with iron oxide deposits for several thousand hours of operation of hot water boilers. In this case, frequent leaks are noted in the pipes of the convective part. The content of iron oxides in sediments usually reaches 80–90%.
Start-up periods are especially important for the operation of hot water boilers. During the initial period of operation at one thermal power plant, oxygen removal was not ensured to the standards established by the PTE. The oxygen content in the make-up water exceeded these standards by 10 times.
The concentration of iron in the make-up water reached 1000 µg/l, and in the return water of the heating network - 3500 µg/l. After the first year of operation, cuttings were made from the network water pipelines; it turned out that their surface contamination with corrosion products was over 2000 g/m2.
It should be noted that at this thermal power plant, before turning on the boiler for operation, the internal surfaces screen pipes and convective beam tubes were chemically cleaned. By the time the samples of screen pipes were cut out, the boiler had worked for 5300 hours. The sample of the screen pipe had an uneven layer of black-brown iron oxide deposits, firmly bound to the metal; height of tubercles 10x12 mm; specific pollution 2303 g/m2.
Sediment composition, %
The metal surface under the layer of deposits was affected by ulcers up to 1 mm deep. Convective beam tubes with inside were covered with iron oxide type deposits of black-brown color with the height of the tubercles up to 3-4 mm. The surface of the metal under the deposits is covered with ulcers various sizes depth 0.3x1.2 and diameter 0.35x0.5 mm. Some tubes had through holes (fistulas).
When hot water boilers are installed in old systems district heating, in which a significant amount of iron oxides have accumulated, cases of deposition of these oxides in the heated boiler pipes are observed. Before turning on the boilers, it is necessary to thoroughly flush the entire system.
A number of researchers recognize the important role in the occurrence of subsludge corrosion of the process of rusting pipes of hot water boilers during their downtime, when proper measures have not been taken to prevent standstill corrosion. Foci of corrosion that arise under the influence of atmospheric air on the wet surfaces of boilers continue to function during operation of the boilers.
The iron-water vapor system is thermodynamically unstable. The interaction of these substances can occur with the formation of magnetite Fe 3 O 4 or wustite FeO:
|
| |
Analysis of reactions (2.1) – (2.3) indicates a peculiar decomposition of water vapor upon interaction with a metal with the formation of molecular hydrogen, which is not a consequence of the actual thermal dissociation of water vapor. From equations (2.1) – (2.3) it follows that during corrosion of steels in superheated steam in the absence of oxygen, only Fe 3 O 4 or FeO can form on the surface.
If there is oxygen in superheated steam (for example, in neutral water conditions, with oxygen dosing into the condensate), hematite Fe 2 O 3 may form in the superheated zone due to the additional oxidation of magnetite.
It is believed that corrosion in steam, starting at a temperature of 570 °C, is chemical. Currently, the maximum superheat temperature for all boilers has been reduced to 545 °C, and, consequently, electrochemical corrosion occurs in superheaters. The outlet sections of primary superheaters are made of corrosion-resistant austenitic of stainless steel, the outlet sections of intermediate superheaters, having the same final superheat temperature (545 °C), are made of pearlitic steels. Corrosion of reheaters is therefore usually severe.
As a result of the action of steam on steel on its initially clean surface, it gradually a so-called topotactic layer is formed, tightly adhered to the metal itself and therefore protecting it from corrosion. Over time, a second so-called epitactic layer grows on this layer. Both of these layers for steam temperatures up to 545 °C are magnetite, but their structure is not the same - the epitactic layer is coarse-grained and does not protect against corrosion.
Steam decomposition rate
mgN 2 /(cm 2 h)

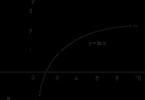
Rice. 2.1. Dependence of the decomposition rate of superheated steam
on wall temperature
Influence the corrosion of overheated surfaces using methods water regime fails. Therefore, the main task of the water-chemical regime of the superheaters themselves is to systematically monitor the state of the metal of the superheaters in order to prevent destruction of the topotactic layer. This can occur due to the entry into the superheaters and the deposition of individual impurities, especially salts, which is possible, for example, as a result of a sharp increase in the level in the drum of high-pressure boilers. The associated salt deposits in the superheater can lead to both an increase in the wall temperature and the destruction of the protective oxide topotactic film, as can be judged by a sharp increase in the rate of steam decomposition (Fig. 2.1).
3.3. Corrosion of the feedwater path and condensate lines
A significant part of the corrosion damage to thermal power plant equipment occurs in the feedwater tract, where the metal is in the most severe conditions, the reason for which is the corrosive aggressiveness of chemically treated water, condensate, distillate and mixtures of them in contact with it. At steam turbine power plants, the main source of contamination of feedwater with copper compounds is ammonia corrosion of turbine condensers and low-pressure regenerative heaters, the piping system of which is made of brass.
The feedwater path of a steam turbine power plant can be divided into two main sections: before the thermal deaerator and after it, and the flow conditions in Their corrosion rates are sharply different. Elements of the first section of the feedwater path, located before the deaerator, include pipelines, tanks, condensate pumps, condensate lines and other equipment. A characteristic feature of corrosion of this part of the nutrient tract is the inability to deplete aggressive agents, i.e., carbonic acid and oxygen contained in the water. Due to the continuous supply and movement of new portions of water along the tract, their loss is constantly replenished. The continuous removal of part of the reaction products of iron with water and the influx of fresh portions of aggressive agents create favorable conditions for intensive corrosion processes.
The source of oxygen in turbine condensate is air suction in the tail part of the turbines and in the seals of condensate pumps. Heating water containing O 2 and CO 2 in surface heaters located in the first section of the supply tract, up to 60–80 °C and above leads to serious corrosion damage to brass pipes. The latter become brittle, and often brass, after several months of operation, acquires a spongy structure as a result of pronounced selective corrosion.
Elements of the second section of the feedwater path - from the deaerator to the steam generator - include feed pumps and lines, regenerative heaters and economizers. The water temperature in this section, as a result of sequential heating of water in regenerative heaters and water economizers, approaches the temperature of the boiler water. The cause of corrosion of equipment related to this part of the tract is mainly the effect on the metal of free carbon dioxide dissolved in the feed water, the source of which is additional chemically treated water. At an increased concentration of hydrogen ions (pH< 7,0), обусловленной наличием растворенной углекислоты и значительным подогревом воды, процесс коррозии на этом участке питательного тракта развивается преимущественно с выделением водорода. Коррозия имеет сравнительно равномерный характер.
In the presence of equipment made of brass (low pressure heaters, condensers), the enrichment of water with copper compounds through the steam-condensate path occurs in the presence of oxygen and free ammonia. An increase in the solubility of hydrated copper oxide occurs due to the formation of copper-ammonia complexes, for example Cu(NH 3) 4 (OH) 2. These products corrode brass heater tubes low pressure begin to decompose in sections of the tract of regenerative high-pressure heaters (HPR) with the formation of less soluble copper oxides, partially deposited on the surface of HPR tubes. d. Cuprous deposits on p.v. tubes. etc. contribute to their corrosion during operation and long-term parking of equipment without conservation.
If the thermal deaeration of the feed water is not deep enough, pitting corrosion is observed mainly on entrance areas economizers, where oxygen is released due to a noticeable increase in the temperature of the feed water, as well as in stagnant areas of the feed tract.
The heat-using equipment of steam consumers and the pipelines through which production condensate is returned to the thermal power plant are subject to corrosion under the influence of the oxygen and carbonic acid it contains. The appearance of oxygen is explained by the contact of condensate with air in open tanks (with open circuit condensate collection) and leaks through leaks in the equipment.
The main measures to prevent corrosion of equipment located in the first section of the feedwater tract (from the water treatment plant to the thermal deaerator) are:
1) the use of protective anti-corrosion coatings on the surfaces of water treatment equipment and tank facilities, which are washed with solutions of acidic reagents or corrosive waters using rubber, epoxy resins, perchlorovinyl-based varnishes, liquid nayrite and silicone;
2) the use of acid-resistant pipes and fittings made of polymer materials (polyethylene, polyisobutylene, polypropylene, etc.) or steel pipes and fittings lined inside with protective coatings applied by flame spraying;
3) the use of heat exchanger pipes made of corrosion-resistant metals (red copper, stainless steel);
4) removal of free carbon dioxide from additional chemically treated water;
5) constant removal of non-condensable gases (oxygen and carbonic acid) from the steam chambers of low-pressure regenerative heaters, coolers and network water heaters and rapid removal of the condensate formed in them;
6) careful sealing of the seals of condensate pumps, fittings and flange connections of supply pipelines under vacuum;
7) ensuring sufficient tightness of turbine condensers on the cooling water and air side and monitoring air suction using recording oxygen meters;
8) equipping condensers with special degassing devices to remove oxygen from the condensate.
To successfully combat corrosion of equipment and pipelines located in the second section of the feedwater path (from thermal deaerators to steam generators), the following measures are applied:
1) equipping thermal power plants with thermal deaerators that produce deaerated water with a residual oxygen and carbon dioxide content under any operating conditions that does not exceed permissible standards;
2) maximum removal of non-condensable gases from the steam chambers of high-pressure regenerative heaters;
3) the use of corrosion-resistant metals for the manufacture of elements of feed pumps in contact with water;
4) anti-corrosion protection of feed and drainage tanks by applying non-metallic coatings that are resistant at temperatures up to 80–100 ° C, for example asbovinyl (a mixture of ethinol varnish with asbestos) or paint and varnish materials based on epoxy resins;
5) selection of corrosion-resistant structural metals suitable for the manufacture of pipes for high-pressure regenerative heaters;
6) constant treatment of feed water with alkaline reagents in order to maintain a given optimal value Feed water pH, at which carbon dioxide corrosion is suppressed and sufficient strength of the protective film is ensured;
7) constant treatment of feed water with hydrazine to bind residual oxygen after thermal deaerators and create an inhibitory effect to inhibit the transition of iron compounds from the surface of the equipment into feed water;
8) sealing the feedwater tanks by organizing a so-called closed system to prevent oxygen from entering the steam generator economizers with the feedwater;
9) implementation of reliable conservation of the equipment of the feedwater path during its downtime in reserve.
An effective method of reducing the concentration of corrosion products in condensate returned to thermal power plants by steam consumers is the introduction of film-forming amines - octadecylamine or its substitutes - into the selected turbine steam sent to consumers. At a concentration of these substances in steam equal to 2–3 mg/dm 3 , it is possible to reduce the content of iron oxides in production condensate by 10–15 times. Dosing of an aqueous emulsion of polyamines using a dosing pump does not depend on the concentration of carbonic acid in the condensate, since their effect is not related to neutralizing properties, but is based on the ability of these amines to form insoluble and non-water-wettable films on the surface of steel, brass and other metals.
Introduction
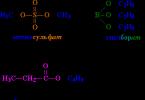
Corrosion (from Latin corrosio - corrosion) is the spontaneous destruction of metals as a result of chemical or physical-chemical interaction with environment. In general, this is the destruction of any material - be it metal or ceramics, wood or polymer. The cause of corrosion is thermodynamic instability construction materials to the effects of substances in the environment in contact with them. Example - oxygen corrosion of iron in water:
4Fe + 2H 2 O + ZO 2 = 2 (Fe 2 O 3 H 2 O)
IN Everyday life For iron alloys (steels), the term “rusting” is more often used. Cases of corrosion of polymers are less known. In relation to them, there is the concept of “aging”, similar to the term “corrosion” for metals. For example, the aging of rubber due to interaction with atmospheric oxygen or the destruction of some plastics under the influence of precipitation, as well as biological corrosion. The rate of corrosion, like any other chemical reaction depends very much on temperature. An increase in temperature of 100 degrees can increase the corrosion rate by several orders of magnitude.
Corrosion processes are characterized by a wide distribution and variety of conditions and environments in which it occurs. Therefore, there is no single and comprehensive classification of corrosion cases encountered. The main classification is made according to the mechanism of the process. There are two types: chemical corrosion and electrochemical corrosion. IN this essay Chemical corrosion is discussed in detail using the example of small and large-capacity ship boiler plants.
Corrosion processes are characterized by a wide distribution and variety of conditions and environments in which it occurs. Therefore, there is no single and comprehensive classification of corrosion cases encountered.
Type aggressive environments, in which the destruction process occurs, corrosion can be of the following types:
1) -Gas corrosion
2) - Corrosion in non-electrolytes
3) -Atmospheric corrosion
4) -Corrosion in electrolytes
5) -Underground corrosion
6) -Biocorrosion
7) - Corrosion by stray current.
According to the conditions of the corrosion process, the following types are distinguished:
1) - Contact corrosion
2) - Crevice corrosion
3) -Corrosion during partial immersion
4) -Corrosion during full immersion
5) -Corrosion during alternating immersion
6) -Friction corrosion
7) -Stress corrosion.
By nature of destruction:
Complete corrosion covering the entire surface:
1) - uniform;
2) - uneven;
3) -selective.
Local (local) corrosion covering individual areas:
1) - spots;
2) - ulcerative;
3) - spot (or pitting);
4) - through;
5) - intercrystalline.
Let's imagine metal in the process of producing rolled metal at a metallurgical plant: a red-hot mass moves along the stands of a rolling mill. Fiery splashes fly out from her in all directions. This is when particles of scale break off from the surface of the metal - a product of chemical corrosion resulting from the interaction of the metal with atmospheric oxygen. This process of spontaneous destruction of a metal due to the direct interaction of oxidizer particles and the oxidized metal is called chemical corrosion.
Chemical corrosion is the interaction of a metal surface with a (corrosive) environment, not accompanied by the occurrence of electrochemical processes at the phase boundary. In this case, the interactions of metal oxidation and reduction of the oxidizing component of the corrosive environment occur in one act. For example, the formation of scale when iron-based materials react at high temperatures with oxygen:
4Fe + 3O 2 → 2Fe 2 O 3
During electrochemical corrosion, the ionization of metal atoms and the reduction of the oxidizing component of the corrosive environment do not occur in one act and their rates depend on the electrode potential of the metal (for example, rusting of steel in sea water).
In chemical corrosion, metal oxidation and reduction of the oxidizing component of the corrosive environment occur simultaneously. Such corrosion is observed when metals are exposed to dry gases (air, fuel combustion products) and liquid non-electrolytes (oil, gasoline, etc.) and is a heterogeneous chemical reaction.
The process of chemical corrosion occurs as follows. The oxidizing component of the external environment, taking away valence electrons from the metal, simultaneously enters into a chemical compound with it, forming a film (corrosion product) on the metal surface. Further formation of the film occurs due to mutual two-way diffusion through the film of the aggressive medium towards the metal and metal atoms towards external environment and their interactions. Moreover, if the resulting film has protective properties, i.e., it prevents the diffusion of atoms, then corrosion proceeds with self-braking over time. Such a film is formed on copper at a heating temperature of 100 °C, on nickel at 650, on iron at 400 °C. Heating steel products above 600 °C leads to the formation of a loose film on their surface. With increasing temperature, the oxidation process accelerates.
The most common type of chemical corrosion is the corrosion of metals in gases at high temperatures - gas corrosion. Examples of such corrosion are oxidation of furnace fittings and engine parts internal combustion, grate bars, parts of kerosene lamps and oxidation during high-temperature processing of metals (forging, rolling, stamping). Other corrosion products may also form on the surface of metal products. For example, when exposed to sulfur compounds, sulfur compounds are formed on iron; on silver, when exposed to iodine vapor, silver iodide is formed, etc. However, most often a layer of oxide compounds is formed on the surface of metals.
Temperature has a great influence on the rate of chemical corrosion. As temperature increases, the rate of gas corrosion increases. The composition of the gas environment has a specific effect on the corrosion rate various metals. Thus, nickel is stable in an environment of oxygen and carbon dioxide, but is highly corroded in an atmosphere of sulfur dioxide. Copper is susceptible to corrosion in an oxygen atmosphere, but is stable in a sulfur dioxide atmosphere. Chromium is corrosion resistant in all three gas environments.
To protect against gas corrosion, heat-resistant alloying with chromium, aluminum and silicon is used, creating protective atmospheres and protective coatings aluminum, chromium, silicon and heat-resistant enamels.
2. Chemical corrosion in ship steam boilers.
Types of corrosion. During operation, the elements of a steam boiler are exposed to aggressive media - water, steam and flue gases. There are chemical and electrochemical corrosion.
Parts and components of machines operating at high temperatures, - piston and turbine engines, rocket engines etc. The chemical affinity of most metals for oxygen at high temperatures is almost unlimited, since the oxides of all technically important metals are able to dissolve in metals and leave the equilibrium system:
2Me(t) + O 2 (g) 2MeO(t); MeO(t) [MeO] (solution)Under these conditions, oxidation is always possible, but along with the dissolution of the oxide, an oxide layer also appears on the surface of the metal, which can inhibit the oxidation process.
The rate of metal oxidation depends on the rate of the chemical reaction itself and the rate of diffusion of the oxidizing agent through the film, and therefore protective effect The better the continuity of the film and the lower the diffusion capacity, the higher it is. The continuity of the film formed on the surface of the metal can be assessed by the ratio of the volume of the formed oxide or some other compound to the volume of the metal spent on the formation of this oxide (Pilling-Badwords factor). Coefficient a (Pilling-Badwords factor) y different metals It has different meanings. Metals that have a<1, не могут создавать сплошные оксидные слои, и через несплошности в слое (трещины) кислород свободно проникает к поверхности металла.
Continuous and stable oxide layers are formed at a = 1.2-1.6, but at large values of a the films are not continuous, easily separated from the metal surface (iron scale) as a result of internal stresses.
The Pilling-Badwords factor gives a very approximate estimate, since the composition of the oxide layers has a wide range of homogeneity, which is also reflected in the density of the oxide. So, for example, for chromium a = 2.02 (for pure phases), but the oxide film formed on it is very resistant to environmental influences. The thickness of the oxide film on the metal surface varies depending on time.
Chemical corrosion, caused by steam or water, destroys the metal evenly over the entire surface. The rate of such corrosion in modern marine boilers is low. More dangerous is local chemical corrosion caused by aggressive chemical compounds contained in ash deposits (sulfur, vanadium oxides, etc.).
Electrochemical corrosion, as its name indicates, is associated not only with chemical processes, but also with the movement of electrons in interacting media, i.e. with the appearance of electric current. These processes occur when the metal interacts with electrolyte solutions, which takes place in a steam boiler in which boiler water circulates, which is a solution of salts and alkalis that have disintegrated into ions. Electrochemical corrosion also occurs when the metal comes into contact with air (at normal temperature), which always contains water vapor, which condenses on the surface of the metal in the form of a thin film of moisture, creating conditions for electrochemical corrosion to occur.

 ;
;